Figure 6.
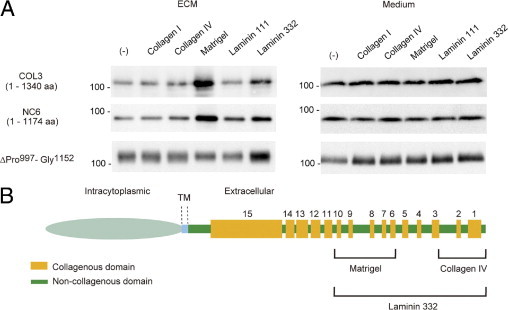
Differential interactions of Ecto-COLXVII with the ECM. The integration of the ectodomain into the matrix depends on the culture substrate. A: Immunoblot of the ECM and culture medium of cells expressing the deletion mutants COL3, NC6, and ΔPro997-Gly1152 cultured on different ECM proteins. Ecto-COLXVII was detected in the ECM by the Ab HK139 and in culture medium by the Ab NC16A-3. Coating with laminin 332 increased binding of the ectodomain of all mutants. In contrast, increased binding to collagen IV was observed only with ΔPro997-Gly1152. This, together with the result shown in Figure 4A, indicates that binding of collagen XVII to collagen IV is mainly mediated by the carboxyl terminus stretching from Ile1341 to Pro1497. On Matrigel, incorporation of the ectodomain of ΔPro997-Gly1152 into the ECM was significantly lower than was that of the other mutants, suggesting that the limited region of Pro997 to Gly1152 is important for binding of collagen XVII to Matrigel. B: Schematic representation of putative interaction sites in the Ecto-ColXVII with different ECM proteins. aa, amino acid; TM, transmembrane.
