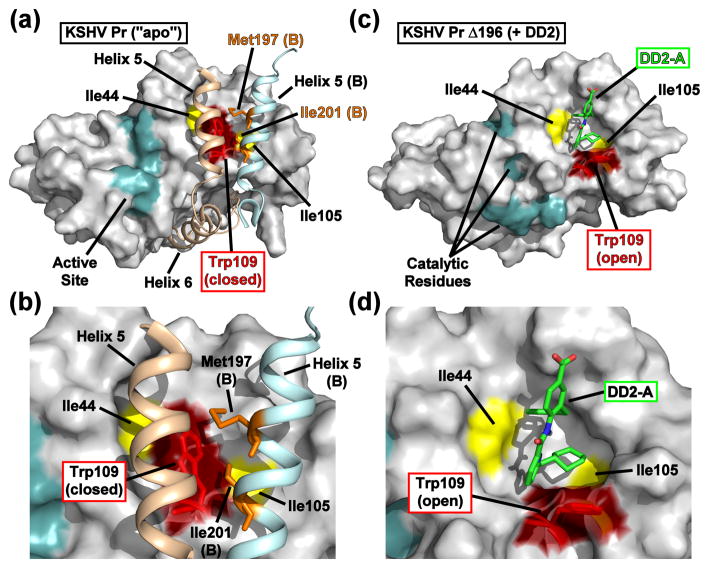
Fig. 4. Comparison of the apo and DD2-bound KSHV Pr crystal structures.
(a) The dimer interface of KSHV Pr (2PBK) consists of two helices from each monomeric unit (helix 5, tan, monomer A; light blue, monomer B), which stabilize the active site (cyan) via the C-terminal helix 6 and occlude the Trp109 (red). (b) The Met197 and Ile201 sidechains from helix 5 of monomer B (orange) form hydrophobic interactions with Trp109 of monomer A. Both Δ196-DD2 monomer A (c) and monomer B (Fig. S5) exhibit independent DD2 binding pockets in which the Trp109 sidechain indole ring (red) adopts an “open” form (d). See also Movie S2.