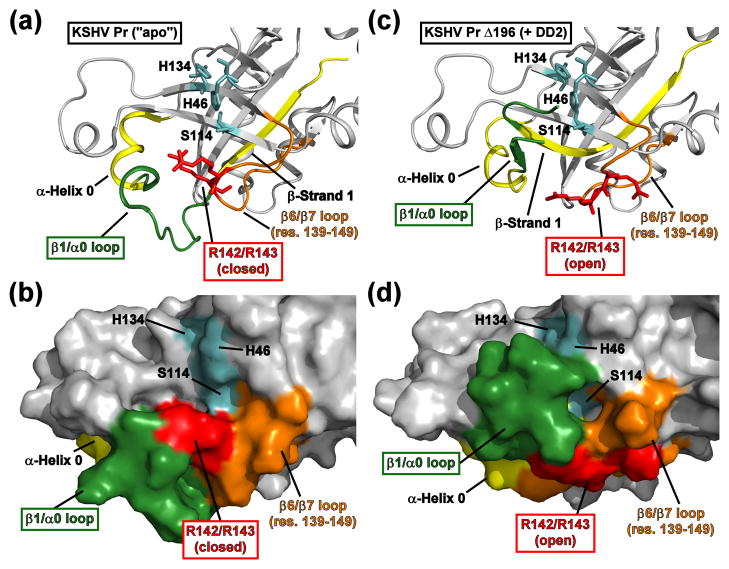
Fig. 7. Structural perturbation of the KSHV Pr active site upon DD2 binding.
(a-b) The active site of the 2PBK represents an “apo” state of KSHV Pr. The catalytic triad (H46, H134, and S114, cyan) and the conserved oxyanion hole-stabilizing arginine residues (R142 and R143, red) are displayed as sticks. Also highlighted are the positions of β-strand 1 and α-helix 0 (yellow), the β1/α0 loop (dark green), and the β6/β7 loop (orange). Residues 197–230 are omitted for clarity. (c-d) The conformation of the “apo” state active site residues displays clear differences relative to the Δ196-DD2 complex (3NJQ). The Arg142 and Arg143 sidechains (red) adopt a “closed” conformation in the apo state, but an “open” conformation while in complex with DD2. In the DD2-bound state, the β1/α0 loop (dark green) occludes the catalytic triad (cyan) and disrupts the substrate binding pocket. See also Movie S3.