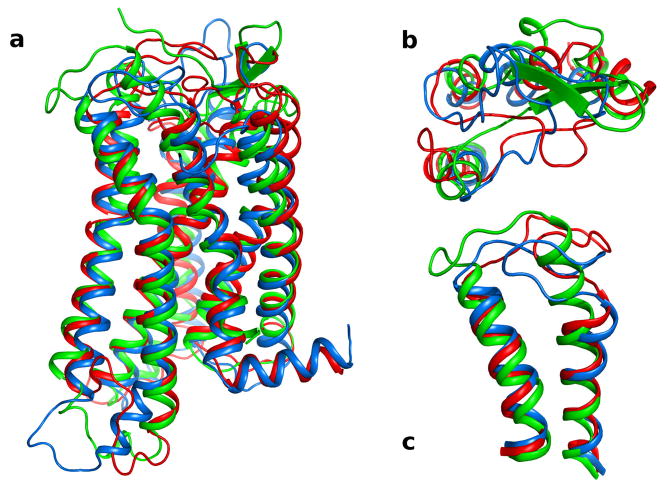
Fig. 2.
Superimposition of three models of the P2Y12R based on different GPCR structural templates. The TM domains of P2Y12R-RHO (red), P2Y12R -A2A (blue), and P2Y12R -CXC (green) were superimposed by the Cα atoms of the residues. Panel a: side view of the Cα ribbon of the P2Y12R models with the extracellular side on the top of the panel. Panel b: the upper part of TMs 2,3,4, and 5, and EL1 and EL2 are shown viewed from the extracellular side highlighting the different conformation assumed by EL1 and EL2 in the three P2Y12R models. Panel c: the extracellular regions of TM6, TM7 and EL3 are shown from the side view. In P2Y12R-CXC, TM7 is two α-helical turn longer and EL3 is shorter compared to TM7 and EL3 in P2Y12R-RHO and P2Y12R-A2A.