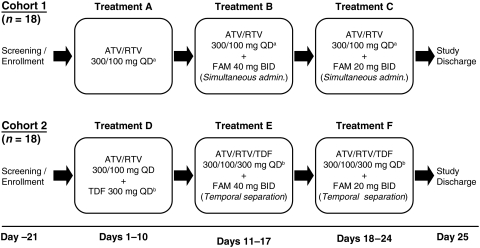
FIG. 1.
Study design. In cohort 1 the morning dose of FAM was administered simultaneously with ATV/RTV and subjects received the following treatments orally: A: Morning dosing of ATV/RTV 300/100 mg QD for 10 days (days 1–10). B: Morning dosing of ATV/RTV 300/100 mg QD+FAM 40 mg twice daily for 7 days (days 11–17). C: Morning dosing of ATV/RTV 300/100 mg QD+FAM 20 mg twice daily for 7 days (days 18–24). In cohort 2 ATV/RTV/TDF was temporarily separated from FAM by administering 2 h before morning FAM and 10 h after afternoon FAM, and subjects received the following treatments orally: D: Morning dosing of ATV/RTV/TDF 300/100/300 mg QD for 10 days (days 1–10); E: Morning dosing of ATV/RTV/TDF 300/100/300 mg QD+FAM 40 mg twice daily for 7 days (days 11–17); and F: Morning dosing of ATV/RTV/TDF 300/100/300 mg QD+FAM 20 mg twice daily for 7 days (days 18–24).+Treatments A, B, and C included ≥2 NRTIs other than TDF.±Treatments D, E, and F included≥1 NRTIs other than TDF. ATV, atazanavir; FAM, famotidine; QD, once daily; RTV, ritonavir.