Abstract
Bipolar cells play a vital role in the transfer of visual information across the vertebrate retina. The synaptic output of these neurons is regulated by factors that are extrinsic and intrinsic. Relatively little is known about the intrinsic factors that regulate neurotransmitter exocytosis. Much of what we know about intrinsic presynaptic mechanisms that regulate glutamate release has come from the study of the unusually large and accessible synaptic terminal of the goldfish rod-dominant bipolar cell, the Mb1 bipolar cell. However, over the past several years, examination of presynaptic mechanisms governing neurotransmitter release has been extended to the mammalian rod bipolar cell. In this review, we discuss the recent advances in our understanding of synaptic vesicle dynamics and neurotransmitter release in rodent rod bipolar cells and consider how these properties help shape the synaptic output of the mammalian retina.
Keywords: rod bipolar cell, exocytosis, endocytosis, ribbon synapse, synaptic vesicle
Introduction
In the vertebrate retina, bipolar cells provide the major pathway through which visual information encoded by photoreceptors is transmitted from the outer retina to the inner retina and ultimately, to the output neurons of the retina, the ganglion cells. While their dendrites reside in the outer plexiform layer, the synaptic terminals of bipolar cells lie in the inner plexiform layer (Figure 1A), where they are exposed to a variety of circuit interactions that modulate synaptic output. The large and accessible terminals of the rod-dominant Mb1 bipolar cells of the goldfish retina have proven an excellent model system for studying presynaptic mechanisms. Data obtained from these neurons have helped lay a foundation for our current understanding of presynaptic mechanisms, from calcium entry and the roles of presynaptic calcium, to synaptic vesicle trafficking and neurotransmitter exocytosis, and the role of the synaptic ribbon. Recently, several groups have extended the study of presynaptic mechanisms to the rod bipolar cell of the rodent retina, which has a different connectivity than the Mb1 bipolar cell and experiences different synaptic demands.
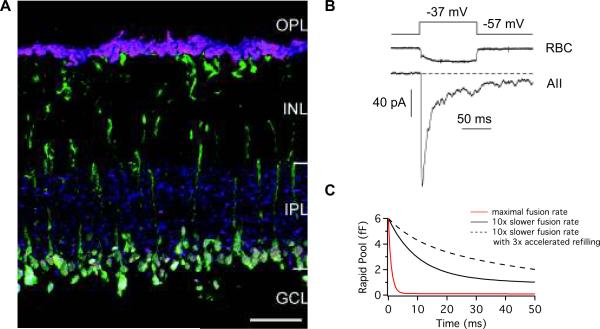
Figure 1. Rod bipolar cells of the mammalian retina.
A) A vertical section of a mouse retina tripled-labeled for PKC-α (a rod bipolar cell marker; green), SV2 (a synaptic vesicle protein; red), and VGluT1 (a vesicular glutamate transporter; blue). A single optical section is shown. VGluT1 and SV2 are selectively found in the outer and inner plexiform layers (OPL and IPL), as expected for proteins located on synaptic vesicles. Photoreceptor terminals, which label for SV2 and VGLuT1 but not PKC-α, are seen as magenta. Rod bipolar cell dendrites, somata and axons, which express PKC-α, are seen as green. Rod bipolar cell terminals, located in the distal IPL, express all three markers and are seen as white. In the IPL, not all SV2 puncta co-localize with VGluT1 in the IPL, consistent with the presence of both glutamatergic and non-glutamatergic synapses. Scale bar: 20 μm.
B) A paired recording from a synaptically-coupled rod bipolar and AII amacrine cell in a retinal slice from the mouse retina. A 100 ms depolarization evokes a sustained Ca2+ current in the presynaptic rod bipolar cell. The postsynaptic AII amacrine cell EPSC exhibits both transient and sustained components. Journal of Physiology 587/11 by Snellman et al., 2009 by John Wiley and Sons reproduced with permission from John Wiley and Sons via Copyright Clearance Center. C) A simple two-step simulation (Innocenti & Heidelberger, 2008) depicting the occupancy state of the rapid vesicle pool, which underlies the transient component of the EPSC. With a maximal fusion rate of 1000 s−1, the rapid pool is quickly depleted (red line), consistent with a prominent EPSC transient component. With a fusion rate of 100 s−1, the depletion of the rapid pool is slower (black line), suggestive of a smaller, broader EPSC transient. Increasing the refilling rate, as might occur when increasing the temperature from ambient room temperature to physiological temperature (Dinkelacker et al., 2000; Pyott & Rosenmund, 2002; Kushmerick et al., 2006), reduces the speed with which the rapid pool is depleted. Dashed line shows a simulation with a release rate of 100 s−1 and a three-times faster refilling rate than that of the solid black line.
Here, we review the current literature on synaptic signaling and neurotransmitter release in the mammalian rod bipolar cell. We compare and contrast results obtained from paired recordings of synaptically-connected rod bipolar cells and third-order neurons, which report on transmitter release detected by postsynaptic glutamate receptors, with those obtained using membrane capacitance measurements, which provide a direct, presynaptic assay of synaptic vesicle fusion. Where instructive, properties of the mammalian rod bipolar cell will be compared with those of the well-studied rod-dominant Mb1 bipolar cell of the goldfish retina.
Basic Physiology and Synaptic Organization
The mammalian rod bipolar cell is part of a high-sensitivity neural pathway specialized for scotopic vision. In contrast to the Mb1 bipolar cell which receives inputs from both rod and cone photoreceptors and makes chemical synapses directly onto third-order neurons (Stell et al., 1977; Ishida et al., 1980; Joselevitch & Kamermans, 2007), the mammalian rod bipolar cell typically receives inputs exclusively from rod photoreceptors (but see also Pang et al., 2010) and indirectly provides outputs to ganglion cells via a unique mammalian circuit between the rod bipolar cell, AII amacrine cells, and cone bipolar cells. This circuit, which grafts the rod pathway onto the cone pathway at the level of cone bipolar cell axon terminals (Kolb & Famiglietti, 1974; Kolb, 1979; Bloomfield & Dacheux, 2001; Tsukamoto et al., 2001; Veruki & Hartveit, 2002), is essential for mammalian scotopic vision. Defects in this pathway are associated with loss of rod-driven, on-center responses in ganglion cells (Deans et al., 2002) and night vision deficits in humans (Berson & Lessell, 1988; Alexander et al., 1992; Bech-Hansen et al., 1998; Strom et al., 1998; see also Fitzgerald et al., 1994).
The visual response of bipolar cells is predominantly sustained (Nelson, 1982; Saito & Kujiraoka, 1982; Kolb & Nelson, 1983; Dacheux & Raviola, 1986; Bloomfield & Xin, 2000; Euler & Masland, 2000; Pang et al., 2004; Joselevitch & Kamermans, 2007); these neurons encode changes in illumination with a graded change in membrane potential (Dowling & Werblin, 1969; Kaneko, 1973), although under certain conditions, the light response might become regenerative (Burrone & Lagnado, 1997; Zenisek & Matthews, 1998; Protti et al., 2000; Ma & Pan, 2003; Palmer, 2006; Hu et al., 2009). In contrast to the largely sustained light response of the rod bipolar cell, the light response of the postsynaptic AII amacrine cell exhibits a mixture of transient and sustained components (Nelson, 1982; Dacheux & Raviola, 1986; Bloomfield & Dacheux, 2001). While the transient transformation may be sculpted by local circuit interactions such as GABAergic feedback (Tachibana & Kaneko, 1987; Maguire et al., 1989; Heidelberger & Matthews, 1991; Maple & Wu, 1998; Vigh & von Gersdoff, 2005; Eggers & Lukasiewicz, 2006; Chavez et al., 2010), the transient component of the AII amacrine cell light response may also arise from intrinsic properties of rod bipolar cell glutamate release.
Rod bipolar cells release the excitatory neurotransmitter glutamate via calcium-dependent exocytosis at ribbon-style active zones (Dowling & Boycott, 1966; Dowling, 1987). Conventional synapses are rare, although they have been reported in some species (Miller et al., 2001; Midorikawa et al., 2007). The synaptic ribbon tethers synaptic vesicles near sites of fusion. The morphology, molecular architecture and general function of synaptic ribbons, also found in some primary sensory neurons such as photoreceptors, has been the topic of numerous recent reviews (Heidelberger et al., 2005; Sterling & Matthews, 2005; tom Dieck & Brandstatter, 2006; Wu et al., 2007; Schmitz, 2009; Zanazzi & Matthews, 2009). An overall picture emerges in which vesicles that are tethered near the base of a synaptic ribbon and close to the presynaptic plasma membrane, wherein calcium channels and fusion machinery components cluster, contribute to the fastest component of glutamate release. Vesicles located further up the ribbon may contribute to a second, larger and more sustained component of release. Both components of release reflect the fusion of vesicles that have undergone all ATP-dependent priming steps required for fusion (Heidelberger, 1998; Heidelberger et al., 2002a). One or both populations may be refilled from those many vesicles that are not associated with synaptic ribbons but are freely-diffusible in the synaptic terminal cytosol (Zenisek et al., 2003; Holt et al., 2004; LoGiudice et al., 2008). In addition, some release may occur outside of ribbon-style active zones (Midorikawa et al., 2007; Zenisek, 2008), although the magnitude of this release and its role in synaptic communication is not yet clear.
In the Mb1 bipolar cell, exocytosis is triggered via calcium entry through L-type voltage-gated calcium channels (Tachibana & Okada, 1991; Tachibana et al., 1993). These channels are preferentially located in the synaptic terminal (Heidelberger & Matthews, 1992; Tachibana et al., 1993) and have been identified as the Cav1.3 (α(1D)) subunit (Logiudice et al., 2006). This type of L-type calcium channel is less sensitive to dihydropyridine agonists and antagonists, rapidly activates at a relatively negative voltage range, and shows a substantially slower rate of calcium-dependent inactivation than other L-type calcium channels (von Gersdorff & Matthews, 1996; Koschak et al., 2001; Xu & Lipscombe, 2001; Logiudice et al., 2006). The latter properties help maintain graded exocytosis during a prolonged stimulus (Heidelberger & Matthews, 1992; von Gersdorff et al., 1996; Sakaba et al., 1997; Mennerick & Matthews, 1998; Protti & Llano, 1998; Xu & Lipscombe, 2001; Zenisek et al., 2003; Logiudice et al., 2006).
Calcium entry through both L- and T-type calcium channels have been proposed to drive exocytosis in mammalian rod bipolar cells (de la Villa et al., 1998; Hartveit, 1999; Pan, 2000; Pan et al., 2001; Hu et al., 2009). However, the primary source of calcium that triggers release in rats and mice maybe different. In the rat rod bipolar terminal, the T-type calcium currents are prominent (Pan, 2000; Pan et al., 2001; Hu et al., 2009), although the L-type Cav1.2 (α(1C)) and/or Cav1.3 subunit have also been shown to be expressed at low levels (Kamphuis & Hendriksen, 1998; Xu et al., 2002). The T-type calcium current in a rat rod bipolar cell terminal is atypical, as it exhibits both transient and sustained components (Pan, 2000). This channel also has an extremely slow activation and slow time dependent inactivation kinetics (Pan, 2000; Pan et al., 2001; Singer & Diamond, 2003), possibly arising from a splicing variant, mutant or other modification of the Cav3.3 (α(1I)) Ca2+ channel subunit (Pan, 2000; Hu et al., 2009). Although the T-type calcium current was originally suggested to drive the transient release of glutamate in the rat rod bipolar cell (Pan et al., 2001), a subsequent study reported transient release in the presence of sustained calcium influx when T-type channels were not activated (Singer and Diamond, 2003). Activation of T-type calcium channels resulted in larger postsynaptic currents at the rat rod bipolar cell-AII amacrine cell synapse, consistent with a role in boosting glutamatergic synaptic transmission (Singer and Diamond, 2003).
In mouse rod bipolar cell terminal, the L-type Cav1.2, Cav1.3 and Cav1.4 (α(1F)) channels are expressed (Satoh et al., 1998; Berntson et al., 2003; Baumann et al., 2004). The major subunit, however, is Cav1.4 (Berntson et al., 2003; Baumann et al., 2004), which shows slow voltage-dependent inactivation and lacks calcium-dependent calcium channel inactivation (Koschak et al., 2003; Baumann et al., 2004; McRory et al., 2004; Wahl-Schott et al., 2006). The properties of this channel are ideal for supporting continuous calcium signaling during prolonged depolarization in the light. Mutations in Cav1.4 channels, which are also present on rod photoreceptor terminals (Morgans et al., 2005), are associated with incomplete X-linked congenital stationary night blindness in humans (CSNB2) (Bech-Hansen et al., 1998; Strom et al., 1998). Mouse bipolar cells have also been reported to express a transient T-type ICa (Kaneko et al., 1989; Kaneko et al., 1991) but there is evidence to suggest that these channels are not present in the synaptic endings (Satoh et al., 1998). Thus, in contrast to the rat, the T-type calcium channel may not be positioned in the mouse rod bipolar cell to drive exocytosis.
Synaptic Vesicle Pools
The concept of synaptic vesicle pools, first introduced in the general exocytosis literature, is based upon the physiological ability to identify distinct components of release. These components possess distinct kinetic properties, magnitudes, and proximity to calcium channels. The latter is evidenced by differential sensitivity to the calcium buffers EGTA and BAPTA, which have similar binding affinities for calcium ions but different on-rates (Adler et al., 1991; Borst & Sakmann, 1996; Mennerick & Matthews, 1996; Sakaba et al., 1997; Moser & Beutner, 2000; Burrone et al., 2002). The naming of these pools has been largely descriptive, with the first pool described in a particular neuron under a particular set of conditions being given the name “releasable pool” or “readily-releasable pool” and subsequent pools being named relative to the first. Thus, some authors have referred to a particular pool as the releasable pool while others have called the same pool the reserve pool depending upon the assay. The commonly used abbreviations for the pools such as “RP” and “RRP” are also confusing as the former may be used to denote either the reserve pool or the releasable pool and the latter, the rapidly-releasing pool or the readily-releasable pool. In the bipolar cell literature, this loose nomenclature has led to some consternation, although in actuality, there is remarkable agreement across laboratories and experimental approaches. Here, we fall back on nomenclature derived from capacitance measurements in other systems and refer to the pool that fuses with properties consistent with vesicles being already docked and primed at the active zone and located closest to calcium channels, as the rapid or rapidly-releasing pool, and the slower releasable pool, which has undergone all ATP-dependent priming steps (Heidelberger, 1998; Heidelberger et al., 2002a), is released within several hundred milliseconds, and is similar in size to the remainder of the ribbon-tethered pool, as the releasable pool. We refer to those vesicles that reside in the cytoplasm, are not attached to synaptic ribbons, and are not recruited to fuse in response to a single one second, maximal depolarization as the cytoplasmic reserve or reserve pool. Properties of the different vesicle pools of rod-driven bipolar cells are summarized in Table 1.
Table 1.
Synaptic vesicle pools of rod-driven bipolar cells
| Mb1 BC | Rat RBC | Mouse RBC | |
|---|---|---|---|
| Ribbon number | 40 – 601 | 362 | 23 – 433,4,5 |
| Vesicle diameter | 29 – 36 nm1,6 | ? | 30 – 50 nm7,8,9 |
| Vesicles per ribbon | ≈ 1101 | 20 – 7010,11 | 12 – 353 |
| Rapid pool | |||
| size (fF) | ≈ 301,12,13 | ≈ 1011 | 7 – 103,9 |
| refilling time constant | τ ≈ 4 – 12 s13,14 τ ≈ 400 ms (a)13 |
τ ≈ 4 s10 τ ≈ 132 ms (b)10 |
? |
| Releasable pool | |||
| size (fF) | 1501,15 | 50 – 6011 | ≈ 303,16 |
| refilling time constant | 8 s17,18,19 | ? | 7 s3 |
| Cytoplasmic reserve pool | |||
| size (# vesicles/cell) | 400,000 – 1,000,0001 | ? | 5,000 – 10,000 (c)7,9 |
| Endocytosis time constant | Fast 1 – 2 s15 Slow 10 – 20 s12,20,21 |
0.7 – 17 s11 | 2 – 21 s3,4,22 |
BC: bipolar cell, RBC: rod bipolar cell. Only key references are included in this table.
Refilling during high Ca2+.
Refilling during ongoing release.
Estimated.
The Rapidly Releasing Pool
A depolarizing voltage step applied to a presynaptic rat rod bipolar cell evokes an excitatory postsynaptic synaptic current (EPSC) in a postsynaptic AII amacrine cell (Figure 1B). This EPSC consists of a prominent fast, transient component followed by a small, more sustained component (Singer & Diamond, 2003; Trexler et al., 2005; Snellman et al., 2009). The transient component was observed under exogenous calcium buffering conditions expected to block all release except that which occurs in the close proximity of an open calcium channel. The activation of this component was limited by the time course of calcium channel activation (Singer & Diamond, 2003), similar to the rapidly-releasing pool of the Mb1 bipolar cell (Mennerick & Matthews, 1996, 1998). The transient component was depleted with a time constant of a few milliseconds (Singer & Diamond, 2006; Oltedal & Hartveit, 2010), also consistent with a rapid pool (Mennerick & Matthews, 1996). Thus, the transient component of the EPSC is likely to result from the exocytosis of neurotransmitter from a limited pool of rapidly-releasing vesicles. The magnitude of the EPSC transient was estimated to reflect the release of glutamate from about 70 vesicles per terminal, or about 7 vesicles per synaptic ribbon, given 10 ribbon synapses between a rod bipolar cell and AII amacrine cell (Singer & Diamond, 2006).
Another way to study synaptic vesicle pools is via membrane capacitance measurements. Membrane capacitance measurements permit changes in surface area indicative of membrane addition and retrieval to be monitored as an index of exocytosis and endocytosis (Lindau & Neher, 1988; Gillis, 1995). Almost a decade ago, Pan and colleagues used this approach in the isolated synaptic terminals of the rat bipolar cell to show that activation of the T-type calcium channel could trigger exocytosis in this neuron (Pan et al., 2001). As with the Mb1 bipolar cell (Mennerick et al., 1997; Palmer et al., 2003), the capacitance approach can be applied to rat rod bipolar cells embedded in a retinal slice provided that the methodology is modified to account for the complex morphology of an intact rat rod bipolar cell (Oltedal et al., 2007; Oltedal & Hartveit, 2010). The capacitance approach has also been successfully used to study synaptic vesicle dynamics in isolated, intact mouse rod bipolar cells under carefully-controlled conditions (Zhou et al., 2006; Wan et al., 2008; Wan et al., 2010). Extra care is warranted when employing capacitance techniques in the mammalian rod bipolar cell when compared with the goldfish Mb1 bipolar cell. This is due to the smaller size of a mammalian rod bipolar cell synaptic terminal, which can hinder direct terminal recordings, particularly in the mouse, and the comparatively long, thin axon connecting the soma and terminal.
Estimates of the magnitude of the rapidly releasing pool made with capacitance measurements are similar between mouse and rat, although that of the mouse is a little smaller. The rapid pool is about 10 fF in the rat (Oltedal & Hartveit, 2010) and approximately 7 fF in mouse (Wan et al., 2008; Wan et al., 2010). As expected for a rapid pool (Mennerick & Matthews, 1996), the rate of exocytosis of this component of release is limited by the activation time course of the presynaptic calcium channels (Oltedal & Hartveit, 2010).
To relate the magnitude of the rapid pool to number of vesicles or vesicles released/synaptic ribbon requires knowledge of the vesicle surface area. Synaptic vesicles in mouse rod bipolar cells have a mean diameter about 30 – 40 nm in fixed tissue (Spiwoks-Becker et al., 2001; LoGiudice et al., 2008; Wan et al., 2010), somewhat larger than that reported for Mb1 bipolar cells (Lagnado et al., 1996; von Gersdorff et al., 1996). If corrected for systematic underestimation in thin sections (Abercrombie, 1946), synaptic vesicle diameter in the mouse rod bipolar cell rises to about 50 nm (Wan et al., 2010). If a specific membrane capacitance of 9 fF/μm2 is assumed (Gentet et al., 2000), a 7 – 10 fF rapid pool would represent about 100 – 400 vesicles per cell, assuming an average vesicle diameter of 30 – 50 nm. Given that there are approximately 35 synaptic ribbons per rodent rod bipolar cell (Singer et al., 2004; Wan et al., 2008), this corresponds to a rapid pool in the rodent rod bipolar cell of between 3 and 11 vesicles per synaptic ribbon, in general agreement with the estimate of 7 vesicles per synaptic ribbon obtained from analysis of the EPSC transient (Singer & Diamond, 2006).
It is important to note that there is some play in the numbers, particularly with respect to the total number of rapidly-releasing vesicles. Capacitance measurements suggest upwards of 100 vesicles in the rapid pool per cell, while the EPSC transient calculations suggest less than 100. While this discrepancy could reflect the possibility that not all fused vesicles release glutamate or that not all released glutamate is detected by postsynaptic receptors, it may also simply reflect the many assumptions and uncertainties inherent in the calculations. Ultrastructural analysis of the rodent rod bipolar cell synaptic ribbon may provide some answers. Taken as a whole, the data support the hypothesis that the transient component of the EPSC reflects fusion of vesicles that belong to the rapidly-releasing pool of vesicles, although, as will be discussed in a subsequent section, the shape of the transient may be influenced by factors that modulate synaptic vesicle dynamics.
The Releasable Pool
In the Mb1 bipolar cell of the goldfish, a 5:1 relationship between the magnitude of the releasable pool and the rapid pool has been noted (Sterling & Matthews, 2005). This relationship led Singer and Diamond to extrapolate from the magnitude of the EPSC transient, representing the rapid pool, a releasable pool of approximately 35 vesicles in the rat rod bipolar cell (Singer & Diamond, 2006). More recently, the magnitude of the releasable pool has been directly assayed using capacitance measurements in both mouse and rat rod bipolar cells. In the isolated mouse rod bipolar cell, this pool is approximately 30 fF (Zhou et al., 2006; Wan et al., 2008), and like the similarly-characterized releasable pool of the Mb1 bipolar cell and other ribbon-style synapses (von Gersdorff & Matthews, 1994a, 1997; Moser & Beutner, 2000; Heidelberger et al., 2002a; Eisen et al., 2004; Rabl et al., 2005), this pool in the mouse rod bipolar cell was depleted with a time constant of a few hundred milliseconds (Wan et al., 2008). Also as in the Mb1 bipolar cell (Mennerick & Matthews, 1996; von Gersdorff et al., 1996), this pool is roughly five times the ≈ 7 fF estimate of the rapid pool obtained from a pulse-duration plot (Wan et al., 2008). With respect to vesicle number, the results suggest a releasable pool of vesicles between 425 and 1200 vesicles per terminal and between 12 and 35 vesicles per synaptic ribbon, for a 30 – 50 nm range of vesicle diameters (see above). In the rat rod bipolar cell, capacitance measurements indicate a releasable pool of 50 – 60 fF (Oltedal & Hartveit, 2010), which is about five times their estimate of the magnitude of the rat rapid pool obtained with capacitance measurements. The 50–60 fF corresponds to 700 – 2400 vesicles, depending upon the assumed diameter and approximately 20 – 69 vesicles per synaptic ribbon.
It has been suggested that because the upper limit for the estimate of releasable vesicles in the rat rod bipolar cell exceeds five times the 7 vesicle per ribbon rapid pool estimate of Singer et al (2006), this could indicate that more vesicles are fusing than are tethered to synaptic ribbons (Oltedal & Hartveit, 2010). Extra-ribbon release has been reported in optical studies of synaptic vesicle fusion in Mb1 bipolar cells (Zenisek et al., 2004; Coggins et al., 2007; Midorikawa et al., 2007; Zenisek, 2008). However, the magnitude of the proposed extra-ribbon release, and its significance to synaptic signaling is not well-delineated. In addition, there is the added complication that ribbons can detach from the plasma membrane (Zenisek et al., 2004; Midorikawa et al., 2007). Given the assumptions inherent in converting capacitance to vesicle number or postsynaptic responses to the number of fused vesicles per ribbon, it would be prudent to await ultrastructural analysis of a ribbon-style active zone in a rodent rod bipolar cell before firmly concluding that there must be extra-ribbon release based solely upon a comparison of results obtained with different approaches. Taken at face value, the numbers do suggest that the synaptic ribbons of the rat rod bipolar cell may tether roughly twice as many vesicles as that of the mouse rod bipolar cell. It will be interesting to see whether this prediction will be confirmed by ultrastructural examination.
The Cytoplasmic Reserve Pool
In addition to the rapid and releasable pools, bipolar cells also contain a large number of cytoplasmic vesicles that are not tethered to synaptic ribbons (von Gersdorff et al., 1996). These cytoplasmic vesicles are much more mobile than those of the conventional synapse and of ribbon-tethered vesicles (Zenisek et al., 2003; Holt et al., 2004; LoGiudice et al., 2008). This enhanced mobility helps support the quick replenishment of releasable pools (Rea et al., 2004; LoGiudice et al., 2008). The reason underlying enhanced mobility is not clear, but one possibility is that it reflects the ribbon-specific lack of synapsin, a synaptic vesicle phosphoprotein thought to attach vesicles to the actin cytoskeleton (Mandell et al., 1990; Mandell et al., 1992; Von Kriegstein et al., 1999; but see Gaffield & Betz 2007). In the Mb1 bipolar cell, there are approximately 1000 vesicles in the cytoplasmic reserve for every vesicle on a the synaptic ribbon (von Gersdorff et al., 1996). The magnitude of this pool is not yet known for the rod bipolar cell. However, if the density of vesicles per terminal is similar to that of the Mb1 bipolar cell, this would suggest a reserve pool in the mouse rod bipolar cell that is > 100 fold smaller (Logiudice et al., 2009; Wan et al., 2010).
Refilling of Vesicle Pools and the Role of Calcium
In much of the secretory literature, a paired-pulse paradigm is used to study refilling of a depleted pool. The first pulse is of a magnitude and duration known to deplete a particular pool. The second pulse in the pair is given at a variable time point after the first to assess the state of refilling of the previously depleted pool. Using this type of static assay, the releasable pool in the Mb1 bipolar neuron was found to refill with a time constant of approximately 7–8 s (von Gersdorff & Matthews, 1997; Heidelberger et al., 2002a; Hull et al., 2006). Refilling of the rapid pool when assayed with a paired-pulse paradigm also exhibited a time constant of a few seconds (τ ≈ 4 s; Mennerick and Matthews, 1996), reflecting the rate of recovery when calcium channels are closed. However, a small, fast component of refilling with a time constant of a few hundred milliseconds has been noted when examining recovery of the releasable pool; this component has been attributed to the refilling of the rapid pool when calcium channels are open (Mennerick & Matthews, 1996). Consistent with a calcium-dependent mechanism, this component is inhibited by intracellular addition of the calcium buffer EGTA (Gomis et al., 1999; see also Sakaba et al., 1997). Together, these observations support that in the Mb1 bipolar cell, as has been described for other neurons and secretory cells, there is calcium-accelerated refilling pool of the rapidly-releasing pool (von Ruden & Neher, 1993; Dittman & Regehr, 1998; Stevens & Wesseling, 1998; Wang & Kaczmarek, 1998; Gomis et al., 1999; Sakaba & Neher. 2001).
In the mammalian rod bipolar cell, refilling of vesicle pools has also been assayed using a paired-pulse protocol. In the rat, recovery from paired-pulse depression of the rapidly-releasing pool was examined by monitoring the recovery of the transient component of the EPSC. The time constant of recovery is approximately 4 s; refilling was faster following a depleting stimulus of a larger magnitude, suggestive of calcium-accelerated refilling (Singer and Diamond 2006). In the mouse rod bipolar cell, recovery from paired-pulse depression has been assayed using capacitance measurements. Like the Mb1 bipolar cell, the mouse rod bipolar cell releasable pool refills with a time constant of about 7 s (Figure 2A). In addition, a small fast component of refilling is present that may reflect calcium-accelerated filling of the rapid pool (Wan et al., 2008). Consistent with this possibility, in response to a brief stimulus train in which each pulse is of a magnitude sufficient to deplete the rapid pool, the evoked cumulative capacitance increase rises more rapidly and to a higher level when the presynaptic calcium concentration is elevated (Figure 2B; see also Wan et al., 2010). Together, these lines of evidence support the hypothesis that, as in the Mb1 bipolar cell and other neurons, refilling of the rapid pool is accelerated by elevated calcium in the mammalian rod bipolar cell.
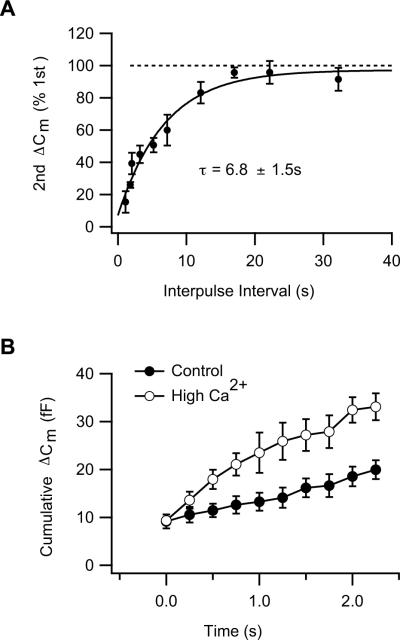
Figure 2. Recovery from depression and the role of presynaptic calcium.
(A) Recovery of the releasable pool from depression was measured in the isolated mouse rod bipolar cell using a paired-pulse paradigm. The second capacitance response as a percentage of the first is plotted against the interpulse interval. The time course of refilling is described by a single exponential function (curve) given by the equation Y=Y0+A1e(−x/τ) where Y0 = 97.3 ± 5.0, A1=90.2 ± 6.3, τ = 6.8 ± 1.5 s. The non-zero intercept is suggests the presence of a fast component of refilling. Reproduced with permission from Wan et al. (2008), copyright Cambridge University Press.
(B) Elevated presynaptic Ca2+ enhances the rate of refilling of the rapid pool in the mouse rod bipolar cell. The cumulative exocytosis, ΔCm, evoked by a brief 4Hz stimulus train (Wan et al., 2010), is plotted as a function of time for each pulse in the train. Each pulse in the train was sufficient to deplete the rapid pool. Filled circles represent data from control cells loaded with standard internal solution (containing 0.5 mM EGTA and no added Ca2+). Open circles represent data from cells loaded with internal solution containing 0.2 mM EGTA and 0.15 mM Ca2+. With the latter solutions, cells had an average resting Ca2+ of ≈ 115 nM compared with ≈ 35 nM in controls and higher stimulus-evoked rises in Ca2+ (Figure 7A, Wan et al., 2010). Data replotted from Wan et al., 2010.
Endocytosis & Synaptic Vesicle recycling
Following exocytosis, fused vesicles are retrieved from the plasma membrane through a process called endocytosis. In neurons, this process is compensatory, meaning that the endocytosis that follows calcium-evoked exocytosis restores the cell surface area back to baseline. Multiple pathways have been suggested to participate in compensatory endocytosis. These include clathrin-mediated retrieval of single vesicles, bulk retrieval of large cisternae, and kiss-and-run retrieval, which is the rapid reversal of a pore-like connection between the vesicle and plasma membrane. The first two, but not the latter, may also require the activity of dynamin, a GTPase essential for membrane scission (Koenig & Ikeda, 1989; Shupliakov et al., 1997; Marks et al., 2001; Yamashita et al., 2005; Lou et al., 2008). Significant advances in our general understanding of endocytosis mechanisms have recently been reviewed (Rizzoli & Betz, 2005; LoGiudice & Matthews, 2007; Wu et al., 2007; Smith et al., 2008; Dittman & Ryan, 2009; Royle & Lagnado, 2010).
In the Mb1 bipolar cell, capacitance studies have identified two modes of compensatory endocytosis. The fast mode of membrane retrieval has a time constant of a few seconds or less, while the slower phase has a time constant in the 10–20 s range (von Gersdorff and Matthews, 1994a; Neves & Lagnado, 1999; Heidelberger et al., 2002b; Hull & von Gersdorff, 2004). Both components may be noted following a single stimulus, and the balance may shift from fast to slow in the presence of elevated presynaptic calcium concentration (von Gersdorff & Matthews, 1994b; Neves & Lagnado, 1999; Heidelberger, 2001). With strong stimuli, a calcium-dependent delay in the onset of endocytosis is also observed (von Gersdorff & Matthews, 1994b; Neves & Lagnado, 1999; Heidelberger 2001).
The fast and slow modes of endocytosis in the Mb1 bipolar cell are thought to reflect distinct mechanistic pathways of membrane retrieval. First, these two pathways are differentially-sensitivity to blockade by elevations in hydrostatic pressure. Elevated hydrostatic pressure selectively inhibits the slow phase of retrieval, sparing the fast (Heidelberger et al., 2002b). Only the slow phase of endocytosis is inhibited by peptides that interfere with clathrin-dependent membrane retrieval (Jockusch et al., 2005). Thus, the slow mode, but not the fast, has been attributed to the clathrin-dependent retrieval of vesicles following their full collapse. The molecular pathway underlying the fast mode is not yet clear. Optical techniques have failed to detect evidence of kiss and run retrieval in the Mb1 bipolar neuron (Zenisek et al., 2002; Llobet et al., 2003), suggesting that the fast mode might represent a mechanism other than kiss and run. The fast mode is selectively inhibited by elevated internal chloride ions (Hull & von Gersdorff, 2004), but like the slow mode, it is requires nucleotide hydrolysis (Heidelberger, 2001; Heidelberger et al., 2002a; Jockusch et al., 2005). Thus, the fast mode of endocytosis may represent dynamin-dependent, clathrin-independent membrane retrieval. Interestingly, introduction of non-hydrolyzable GTP analogs into the Mb1 terminal was found to inhibit both fast and slow modes of endocytosis following a relatively mild stimulus (Jockusch et al., 2005), but not after a stimulus that depleted the entire releasable pool (Heidelberger, 2001). Although other explanations are possible, this differential effect may reflect an activity-dependent transition between dynamin-dependent and dynamin-independent rapid and slow endocytosis, as reported recently in the calyx of Held (Xu et al., 2008). Following very strong or prolonged exocytosis, endocytosis in the Mb1 bipolar cell can also occur by a form of bulk retrieval that does not involve clathrin-coated vesicles or pits (Holt et al., 2003; Paillart et al., 2003). Inhibitors of PI-3-kinase block bulk retrieval, but not the fast and slow modes (Holt et al., 2003), indicating that bulk membrane retrieval may occur by a different biochemical pathway (Clayton et al., 2010).
In the mammalian rod bipolar cell, calcium-evoked exocytosis is followed by compensatory endocytosis. Reminiscent of endocytosis in the Mb1 bipolar neuron, in both the mouse and rat rod bipolar cell, the time course of endocytosis is prolonged with stronger stimuli or high presynaptic calcium loads (Wan et al., 2008; Oltedal & Hartveit, 2010). In addition, the brief delay between the succession of exocytosis and the onset of endocytosis is prolonged with larger presynaptic calcium loads. In response to a single depolarization of a few hundred milliseconds to a second in duration, an average time constant of membrane retrieval of ≈ 7 s was reported in the mouse rod bipolar cell, with a range of 2–21 s depending upon the depolarization-evoked rise in the presynaptic calcium concentration. In rat rod bipolar cell, the time constant of retrieval averaged ≈ 17 s, although values as low as ≈ 1 s were noted, depending upon the stimulus. A separate, fast phase of retrieval was not discernable in either species, although the existence of one cannot be excluded due to resolution issues and the possibility that some amount of exocytosis may occur following calcium channel closure (e.g., Figure 3, inset). We do note that in both species, the time constant of recovery is faster following milder stimulations, (Wan et al., 2008; Oltedal & Hartveit, 2010). These shorter time constants are similar to those of the fast phase of endocytosis reported in other neurons and could indicate the presence a fast mode of endocytosis that is limited in capacity. Future experiments will be needed to resolve this issue.
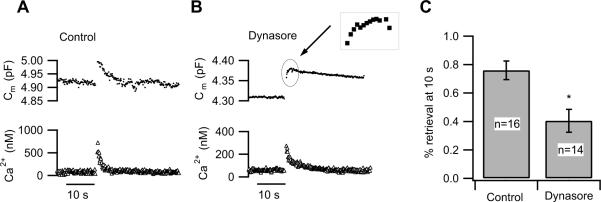
Figure 3. Dynasore inhibits compensatory endocytosis in the mouse rod bipolar cell.
(A) Representative example of exocytosis followed by endocytosis evoked by a 500 ms depolarization in a mouse rod bipolar cell.
(B) Representative example of the response to a 500 ms depolarization in a mouse rod bipolar cell treated with 160 μM dynasore. Note the slow time course of membrane recovery. Inset shows a small rise in membrane capacitance that occurs after the closure of calcium channels.
(C) The extent of membrane retrieval 10 s after calcium channel closure is significantly reduced in rod bipolar cells treated with dynasore (p <0.05). Ten seconds after calcium channel closure, control cells retrieved ≈ 76% of membrane added via stimulus-evoked exocytosis. By contrast, the extent of membrane retrieval in this same interval was reduced to ≈ 40% in cells exposed to dynasore. In 2/14 cells, dynasore fully blocked endocytosis, whereas all control cells exhibited membrane recovery. Error bars represent SEM. (Wan and Heidelberger, unpublished data)
Recently, using a combination of electrophysiology, confocal imaging and electron microscopy, LoGiudice et al. (2009) reported that exocytosis was followed by the retrieval of single, clathrin-coated vesicles. Retrieval was dynamin-dependent, as evidenced by the fact that terminals treated with an potent inhibitor of dynamin function called dynasore had more vesicles attached to the plasma membrane after stimulation (Logiudice et al., 2009). Using membrane capacitance measurements to study membrane retrieval, we have shown directly that compensatory endocytosis is inhibited by dynasore in the mouse rod bipolar cell (Figure 3). Dynasore had no effect on the magnitude of the stimulus-evoked calcium transient or on the recovery of the presynaptic calcium concentration to baseline (not shown), however, resting calcium was mildly elevated in the presence of dynasore (control: 43 ± 9 nM, n=16; dynasore: 83 ± 21 nM, n=14; P = 0.008, Mann-Whitney Rank Sum Test). Whether this elevation is sufficient to impede the recovery of membrane capacitance in the mouse rod bipolar cell is not known, but it is well below the levels that impede endocytosis in the Mb1 bipolar cell (von Gersdorff & Matthews, 1994b; Rouze & Schwartz, 1998). Thus, it seems reasonable to provisionally postulate that the dominant mode of compensatory endocytosis under the conditions tested is both dynamin- and clathrin-dependent in the mammalian rod bipolar cell.
Synaptic vesicle dynamics and the postsynaptic response
It is generally agreed that an understanding of synaptic vesicle dynamics is an essential part of understanding how the pattern of neurotransmitter release is determined and thus, how presynaptic properties dictate the postsynaptic response. For the rod bipolar cell to AII amacrine cell synapse, the evidence presented above suggests that the transient component of the EPSC corresponds to the exocytosis of neurotransmitter from a small, rapid pool of vesicles. The structure of the ribbon, combined with recent optical work (Zenisek et al., 2000; Zenisek et al., 2004; LoGiudice et al., 2008), provides support for the hypothesis that vesicle replenishment at ribbon-style active zones is important for on-going release and that these replacement vesicles can come from higher rows of vesicles tethered to the synaptic ribbons.
A related question is the extent to which the experiments used to probe the vesicle pools recapitulate the light-driven responses of the postsynaptic AII amacrine cells. While the light responses of third-order neurons have both transient and sustained components, the transient component is not nearly as prominent under physiological conditions as it is under the types of experimental protocols presented above. A clue to understanding this apparent discrepancy comes from earlier work in the Mb1 bipolar cell in which a depolarization-dependent delay in glutamate exocytosis was reported (von Gersdorff et al., 1998). Activation of a glutamate-gated current attributable to fusion of vesicles in the rapid pool was more rapid in onset, peaked faster and was larger when evoked by a strong depolarizing voltage step. This current was delayed in onset, peaked more slowly, and was smaller when evoked by a voltage step to a less positive potential (von Gersdorff et al., 1998). Similarly, even when the voltage response of the Mb1 bipolar cell is regenerative, spike latency decreases with light intensity (Protti et al., 2000). Thus, stimulus strength has the ability to alter the kinetics of membrane depolarization, glutamate release and the shape of the EPSC, presumably via its effect on calcium channel activity and associated changes in the presynaptic calcium concentration.
In an elegant study, Snellman and colleagues reflected upon the fact that in most studies of mammalian rod bipolar cell of exocytosis, a depolarizing voltage step was used to evoke calcium-triggered glutamate release. The essentially instantaneous depolarization drives a concerted opening of calcium channels, resulting in a relatively rapid rise in the presynaptic calcium concentration. Because the rate of vesicle fusion is steeply calcium-dependent (Heidelberger et al., 1994), such stimuli will trigger the fast fusion of vesicles in the rapid pool. By contrast, the membrane response of the mammalian rod bipolar cell to light is not instantaneous, but may take 100 ms or more to peak (Euler & Masland, 2000; Berntson et al., 2004; Trexler et al., 2005). Snellman et al. (2009) varied the properties of the stimuli given to the rod bipolar cell and demonstrated that the shape of the AII amacrine cell EPSC is profoundly influenced by the rate at which the rod bipolar cell is depolarized. Faster membrane depolarizations were associated with a pronounced transient component of the EPSC, while slower rates of membrane depolarization favored more sustained release.
The effect of rate can generally be understood by looking at a simple computer simulation of the occupancy state of the rapid pool (Figure 1C). In this simulation, we compare the time course of depletion of the rapid pool when the fusion rate is changed ten-fold, keeping all other starting parameters constant. With a fusion rate comparable to that of a non-neuronal secretory cell or asynchronous release in a neuron (Heinemann et al., 1994; Sun et al., 2007), the rapid pool empties over a period of tens of milliseconds (Figure 1C; black trace; fusion rate = 100 s−1). With a fusion rate of 1000 s−1 (Figure 1C; red trace), which is similar in magnitude to the maximal fusion rates of neurons (Heidelberger et al., 1994; Bollmann et al., 2000; Schneggenburger & Neher, 2000; Beutner et al., 2001; Duncan et al., 2010), the pool becomes depleted within a few milliseconds, indicative of a fast bolus of glutamate release.
In a similar vein, it is important to note that all of the studies on bipolar cell synaptic vesicle dynamics cited above were performed at room temperature. From a mechanistic viewpoint, this facilitates direct comparisons across cells from different species. However, many aspects of synaptic vesicle dynamics may be temperature-sensitive, and thus caution should be used when extrapolating data obtained at room temperature to physiological temperature. For example, studies in other glutamatergic neurons have indicated that the rate of pool refilling is two or three times faster at physiological temperature than at room temperature (Dinkelacker et al., 2000; Pyott & Rosenmund, 2002; Kushmerick et al., 2006). It is therefore conceivable that at physiological temperatures, the rates of pool refilling in the mammalian rod bipolar cell will be faster than those reported above and in Table 1, by a factor of two or three. The simulation in Figure 1C (dashed trace) shows that a three-fold increase in the refilling rate delays the depletion of the rapid pool. The predicted response at physiological temperature would therefore be less transient in nature than that observed at room temperature. While this simulation is overly simplistic, it serves to highlight the importance of both synaptic vesicle dynamics and temperature-dependent kinetics in shaping synaptic output.
Concluding Remarks
Many of the basic features of synaptic vesicle pools and their dynamics have now been characterized for the mammalian rod bipolar cell. The different vesicle pools, their relationship to each other and the interplay between pools, and the kinetics of vesicle transitions all have the ability to sculpt the pattern of neurotransmitter release. An important goal for the future will be to understand the contributions of synaptic vesicle dynamics to neurotransmitter release under physiological conditions. Furthermore, given that several of the steps in the exocytosis pathway are regulated by calcium, a related topic is the extent to which synaptic vesicle dynamics change with changes in background illumination that may alter steady state calcium levels. Might the rod bipolar cell secretory machinery adapt to steady illumination and prolonged elevation of the presynaptic calcium concentration, as has been postulated in a recent study (Wan et al., 2010)? If so, what advantage does such adaptation provide? Does it preserve the gain of the synapse or change it? Finally, the move into the mammalian system, especially the mouse rod bipolar cell, along with recent explorations into zebrafish bipolar cell physiology (reviewed in Connaughton, 2011), will open up the field to the identification and study of presynaptic molecules that modulate the exocytotic pathway, and hence synaptic transmission from bipolar cells to second-and third-order neurons.
Acknowledgments
This work was supported by National Eye Institute Award 5R01EY012128. We thank Alejandro Vila for Figure 1A. We thank Proleta Datta for comments on the manuscript.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec (Hoboken) 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KR, Fishman GA, Peachey NS, Marchese AL, Tso MO. `On' response defect in paraneoplastic night blindness with cutaneous malignant melanoma. Invest Ophthalmol Vis Sci. 1992;33:477–483. [PubMed] [Google Scholar]
- Baumann L, Gerstner A, Zong X, Biel M, Wahl-Schott C. Functional characterization of the L-type Ca2+ channel Cav1.4alpha1 from mouse retina. Invest Ophthalmol Vis Sci. 2004;45:708–713. doi: 10.1167/iovs.03-0937. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- Berntson A, Smith RG, Taylor WR. Transmission of single photon signals through a binary synapse in the mammalian retina. Vis Neurosci. 2004;21:693–702. doi: 10.1017/S0952523804215048. [DOI] [PubMed] [Google Scholar]
- Berntson A, Taylor WR, Morgans CW. Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. J Neurosci Res. 2003;71:146–151. doi: 10.1002/jnr.10459. [DOI] [PubMed] [Google Scholar]
- Berson EL, Lessell S. Paraneoplastic night blindness with malignant melanoma. Am J Ophthalmol. 1988;106:307–311. doi: 10.1016/0002-9394(88)90366-2. [DOI] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. J Physiol. 2000;523(Pt 3):771–783. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Burrone J, Lagnado L. Electrical resonance and Ca2+ influx in the synaptic terminal of depolarizing bipolar cells from the goldfish retina. J Physiol. 1997;505(Pt 3):571–584. doi: 10.1111/j.1469-7793.1997.571ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Neves G, Gomis A, Cooke A, Lagnado L. Endogenous calcium buffers regulate fast exocytosis in the synaptic terminal of retinal bipolar cells. Neuron. 2002;33:101–112. doi: 10.1016/s0896-6273(01)00565-7. [DOI] [PubMed] [Google Scholar]
- Connaughton VP. 2011.
- Chavez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci. 2010;30:2330–2339. doi: 10.1523/JNEUROSCI.5574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Sue N, Smillie KJ, O'Leary T, Bache N, Cheung G, Cole AR, Wyllie DJ, Sutherland C, Robinson PJ, Cousin MA. Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nat Neurosci. 2010;13:845–851. doi: 10.1038/nn.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins MR, Grabner CP, Almers W, Zenisek D. Stimulated exocytosis of endosomes in goldfish retinal bipolar neurons. J Physiol. 2007;584:853–865. doi: 10.1113/jphysiol.2007.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Villa P, Vaquero CF, Kaneko A. Two types of calcium currents of the mouse bipolar cells recorded in the retinal slice preparation. Eur J Neurosci. 1998;10:317–323. doi: 10.1046/j.1460-9568.1998.00051.x. [DOI] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelacker V, Voets T, Neher E, Moser T. The readily releasable pool of vesicles in chromaffin cells is replenished in a temperature-dependent manner and transiently overfills at 37 degrees C. J Neurosci. 2000;20:8377–8383. doi: 10.1523/JNEUROSCI.20-22-08377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. The retina : an approachable part of the brain. Belknap Press of Harvard University Press; Cambridge, Mass: 1987. [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Werblin FS. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969;32:315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- Duncan G, Rabl K, Gemp I, Heidelberger R, Thoreson WB. Quantitative analysis of synaptic release at the photoreceptor synapse. Biophys J. 2010;98:2102–2110. doi: 10.1016/j.bpj.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol. 2006;572:215–225. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MD, Spassova M, Parsons TD. Large releasable pool of synaptic vesicles in chick cochlear hair cells. J Neurophysiol. 2004;91:2422–2428. doi: 10.1152/jn.01130.2003. [DOI] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KM, Cibis GW, Giambrone SA, Harris DJ. Retinal signal transmission in Duchenne muscular dystrophy: evidence for dysfunction in the photoreceptor/depolarizing bipolar cell pathway. J Clin Invest. 1994;93:2425–2430. doi: 10.1172/JCI117250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, Betz WJ. Synaptic vesicle mobility in mouse motor nerve terminals with and without synapsin. J Neurosci. 2007;27:13691–13700. doi: 10.1523/JNEUROSCI.3910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Stuart GJ, Clements JD. Direct measurement of specific membrane capacitance in neurons. Biophys J. 2000;79:314–320. doi: 10.1016/S0006-3495(00)76293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis KD. Techniques for membrane capacitance measurements. In: Neher E, Sakmann B, editors. Single Channel Recording. 2nd edn Plenum Press; New York: 1995. pp. 155–198. [Google Scholar]
- Gomis A, Burrone J, Lagnado L. Two actions of calcium regulate the supply of releasable vesicles at the ribbon synapse of retinal bipolar cells. J Neurosci. 1999;19:6309–6317. doi: 10.1523/JNEUROSCI.19-15-06309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Heidelberger R. Adenosine triphosphate and the late steps in calcium-dependent exocytosis at a ribbon synapse. J Gen Physiol. 1998;111:225–241. doi: 10.1085/jgp.111.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R. ATP is required at an early step in compensatory endocytosis in synaptic terminals. J Neurosci. 2001;21:6467–6474. doi: 10.1523/JNEUROSCI.21-17-06467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Inhibition of calcium influx and calcium current by gamma-aminobutyric acid in single synaptic terminals. Proc Natl Acad Sci U S A. 1991;88:7135–7139. doi: 10.1073/pnas.88.16.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol. 1992;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Sterling P, Matthews G. Roles of ATP in depletion and replenishment of the releasable pool of synaptic vesicles. J Neurophysiol. 2002a;88:98–106. doi: 10.1152/jn.2002.88.1.98. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Thoreson WB, Witkovsky P. Synaptic transmission at retinal ribbon synapses. Prog Retin Eye Res. 2005;24:682–720. doi: 10.1016/j.preteyeres.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Zhou ZY, Matthews G. Multiple components of membrane retrieval in synaptic terminals revealed by changes in hydrostatic pressure. J Neurophysiol. 2002b;88:2509–2517. doi: 10.1152/jn.00267.2002. [DOI] [PubMed] [Google Scholar]
- Heinemann C, Chow RH, Neher E, Zucker RS. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+ Biophys J. 1994;67:2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Cooke A, Neef A, Lagnado L. High mobility of vesicles supports continuous exocytosis at a ribbon synapse. Curr Biol. 2004;14:173–183. doi: 10.1016/j.cub.2003.12.053. [DOI] [PubMed] [Google Scholar]
- Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci. 2003;23:1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Bi A, Pan ZH. Differential expression of three T-type calcium channels in retinal bipolar cells in rats. Vis Neurosci. 2009;26:177–187. doi: 10.1017/S0952523809090026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J Neurophysiol. 2006;96:2025–2033. doi: 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, von Gersdorff H. Fast endocytosis is inhibited by GABA-mediated chloride influx at a presynaptic terminal. Neuron. 2004;44:469–482. doi: 10.1016/j.neuron.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti B, Heidelberger R. Mechanisms contributing to tonic release at the cone photoreceptor ribbon synapse. J Neurophysiol. 2008;99(1):25–36. doi: 10.1152/jn.00737.2007. [DOI] [PubMed] [Google Scholar]
- Ishida AT, Stell WK, Lightfoot DO. Rod and cone inputs to bipolar cells in goldfish retina. J Comp Neurol. 1980;191:315–335. doi: 10.1002/cne.901910302. [DOI] [PubMed] [Google Scholar]
- Jockusch WJ, Praefcke GJ, Mcmahon HT, Lagnado L. Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron. 2005;46:869–878. doi: 10.1016/j.neuron.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Joselevitch C, Kamermans M. Interaction between rod and cone inputs in mixed-input bipolar cells in goldfish retina. J Neurosci Res. 2007;85:1579–1591. doi: 10.1002/jnr.21249. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Hendriksen H. Expression patterns of voltage-dependent calcium channel alpha 1 subunits (alpha 1A-alpha 1E) mRNA in rat retina. Brain Res Mol Brain Res. 1998;55:209–220. doi: 10.1016/s0169-328x(97)00363-x. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973;235:133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Pinto LH, Tachibana M. Transient calcium current of retinal bipolar cells of the mouse. J Physiol. 1989;410:613–629. doi: 10.1113/jphysiol.1989.sp017551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Suzuki S, Pinto LH, Tachibana M. Membrane currents and pharmacology of retinal bipolar cells: a comparative study on goldfish and mouse. Comp Biochem Physiol C. 1991;98:115–127. doi: 10.1016/0742-8413(91)90188-y. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974;186:47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R. Rod pathways in the retina of the cat. Vision Res. 1983;23:301–312. doi: 10.1016/0042-6989(83)90078-0. [DOI] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. Alpha 1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Walter D, Hoda JC, Heinzle T, Grabner M, Striessnig J. Cav1.4alpha1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J Neurosci. 2003;23:6041–6049. doi: 10.1523/JNEUROSCI.23-14-06041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Renden R, von Gersdorff H. Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment. J Neurosci. 2006;26:1366–1377. doi: 10.1523/JNEUROSCI.3889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L, Gomis A, Job C. Continuous vesicle cycling in the synaptic terminal of retinal bipolar cells. Neuron. 1996;17:957–967. doi: 10.1016/s0896-6273(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Llobet A, Beaumont V, Lagnado L. Real-time measurement of exocytosis and endocytosis using interference of light. Neuron. 2003;40:1075–1086. doi: 10.1016/s0896-6273(03)00765-7. [DOI] [PubMed] [Google Scholar]
- Logiudice L, Henry D, Matthews G. Identification of calcium channel alpha1 subunit mRNA expressed in retinal bipolar neurons. Mol Vis. 2006;12:184–189. [PMC free article] [PubMed] [Google Scholar]
- Logiudice L, Matthews G. Endocytosis at ribbon synapses. Traffic. 2007;8:1123–1128. doi: 10.1111/j.1600-0854.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- LoGiudice L, Sterling P, Matthews G. Mobility and turnover of vesicles at the synaptic ribbon. J Neurosci. 2008;28:3150–3158. doi: 10.1523/JNEUROSCI.5753-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logiudice L, Sterling P, Matthews G. Vesicle recycling at ribbon synapses in the finely branched axon terminals of mouse retinal bipolar neurons. Neuroscience. 2009;164:1546–1556. doi: 10.1016/j.neuroscience.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Paradise S, Ferguson SM, De Camilli P. Selective saturation of slow endocytosis at a giant glutamatergic central synapse lacking dynamin 1. Proc Natl Acad Sci U S A. 2008;105:17555–17560. doi: 10.1073/pnas.0809621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YP, Pan ZH. Spontaneous regenerative activity in mammalian retinal bipolar cells: roles of multiple subtypes of voltage-dependent Ca2+ channels. Vis Neurosci. 2003;20:131–139. doi: 10.1017/s0952523803202042. [DOI] [PubMed] [Google Scholar]
- Maguire G, Maple B, Lukasiewicz P, Werblin F. Gamma-aminobutyrate type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamander. Proc Natl Acad Sci U S A. 1989;86:10144–10147. doi: 10.1073/pnas.86.24.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, Czernik AJ, De Camilli P, Greengard P, Townes-Anderson E. Differential expression of synapsins I and II among rat retinal synapses. J Neurosci. 1992;12:1736–1749. doi: 10.1523/JNEUROSCI.12-05-01736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, Townes-Anderson E, Czernik AJ, Cameron R, Greengard P, De Camilli P. Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron. 1990;5:19–33. doi: 10.1016/0896-6273(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Maple BR, Wu SM. Glycinergic synaptic inputs to bipolar cells in the salamander retina. J Physiol. 1998;506(Pt 3):731–744. doi: 10.1111/j.1469-7793.1998.731bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks B, Stowell MH, Vallis Y, Mills IG, Gibson A, Hopkins CR, McMahon HT. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410:231–235. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- Mcrory JE, Hamid J, Doering CJ, Garcia E, Parker R, Hamming K, Chen L, Hildebrand M, Beedle AM, Feldcamp L, Zamponi GW, Snutch TP. The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J Neurosci. 2004;24:1707–1718. doi: 10.1523/JNEUROSCI.4846-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Rapid calcium-current kinetics in synaptic terminals of goldfish retinal bipolar neurons. Vis Neurosci. 1998;15:1051–1056. doi: 10.1017/s0952523898156043. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zenisek D, Matthews G. Static and dynamic membrane properties of large-terminal bipolar cells from goldfish retina: experimental test of a compartment model. J Neurophysiol. 1997;78:51–62. doi: 10.1152/jn.1997.78.1.51. [DOI] [PubMed] [Google Scholar]
- Midorikawa M, Tsukamoto Y, Berglund K, Ishii M, Tachibana M. Different roles of ribbon-associated and ribbon-free active zones in retinal bipolar cells. Nat Neurosci. 2007;10:1268–1276. doi: 10.1038/nn1963. [DOI] [PubMed] [Google Scholar]
- Miller RF, Gottesman J, Henderson D, Sikora M, Kolb H. Pre- and postsynaptic mechanisms of spontaneous, excitatory postsynaptic currents in the salamander retina. Prog Brain Res. 2001;131:241–253. doi: 10.1016/s0079-6123(01)31020-8. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR. Photoreceptor calcium channels: insight from night blindness. Vis Neurosci. 2005;22(5):561–568. doi: 10.1017/S0952523805225038. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol. 1982;47:928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
- Neves G, Lagnado L. The kinetics of exocytosis and endocytosis in the synaptic terminal of goldfish retinal bipolar cells. J Physiol. 1999;515(Pt 1):181–202. doi: 10.1111/j.1469-7793.1999.181ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltedal L, Hartveit E. Transient release kinetics of rod bipolar cells revealed by capacitance measurement of exocytosis from axon terminals in rat retinal slices. J Physiol. 2010;588:1469–1487. doi: 10.1113/jphysiol.2010.186916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltedal L, Morkve SH, Veruki ML, Hartveit E. Patch-clamp investigations and compartmental modeling of rod bipolar axon terminals in an in vitro thin-slice preparation of the mammalian retina. J Neurophysiol. 2007;97:1171–1187. doi: 10.1152/jn.01010.2006. [DOI] [PubMed] [Google Scholar]
- Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci. 2003;23:4092–4099. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ. Modulation of Ca2+-activated K+ currents and Ca2+-dependent action potentials by exocytosis in goldfish bipolar cell terminals. J Physiol. 2006;572:747–762. doi: 10.1113/jphysiol.2006.105205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ, Hull C, Vigh J, von Gersdorff H. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23:11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZH. Differential expression of high- and two types of low-voltage-activated calcium currents in rod and cone bipolar cells of the rat retina. J Neurophysiol. 2000;83:513–527. doi: 10.1152/jn.2000.83.1.513. [DOI] [PubMed] [Google Scholar]
- Pan ZH, Hu HJ, Perring P, Andrade R. T-type Ca2+ channels mediate neurotransmitter release in retinal bipolar cells. Neuron. 2001;32:89–98. doi: 10.1016/s0896-6273(01)00454-8. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Lem J, Bramblett DE, Paul DL, Wu SM. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc Natl Acad Sci U S A. 2010;107:395–400. doi: 10.1073/pnas.0907178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol. 2004;558:897–912. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Flores-Herr N, von Gersdorff H. Light evokes Ca2+ spikes in the axon terminal of a retinal bipolar cell. Neuron. 2000;25:215–227. doi: 10.1016/s0896-6273(00)80884-3. [DOI] [PubMed] [Google Scholar]
- Protti DA, Llano I. Calcium currents and calcium signaling in rod bipolar cells of rat retinal slices. J Neurosci. 1998;18:3715–3724. doi: 10.1523/JNEUROSCI.18-10-03715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott SJ, Rosenmund C. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J Physiol. 2002;539:523–535. doi: 10.1113/jphysiol.2001.013277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl K, Cadetti L, Thoreson WB. Kinetics of exocytosis is faster in cones than in rods. J Neurosci. 2005;25:4633–4640. doi: 10.1523/JNEUROSCI.4298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea R, Li J, Dharia A, Levitan ES, Sterling P, Kramer RH. Streamlined synaptic vesicle cycle in cone photoreceptor terminals. Neuron. 2004;41:755–766. doi: 10.1016/s0896-6273(04)00088-1. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Rouze NC, Schwartz EA. Continuous and transient vesicle cycling at a ribbon synapse. J Neurosci. 1998;18:8614–8624. doi: 10.1523/JNEUROSCI.18-21-08614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle SJ, Lagnado L. Clathrin-mediated endocytosis at the synaptic terminal: bridging the gap between physiology and molecules. Traffic. 2010 doi: 10.1111/j.1600-0854.2010.01104.x. [Epub ahead of print], published online: 3 AUG 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Kujiraoka T. Physiological and morphological identification of two types of on-center bipolar cells in the carp retina. J Comp Neurol. 1982;205:161–170. doi: 10.1002/cne.902050207. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32(6):1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Tachibana M, Matsui K, Minami N. Two components of transmitter release in retinal bipolar cells: exocytosis and mobilization of synaptic vesicles. Neurosci Res. 1997;27:357–370. doi: 10.1016/s0168-0102(97)01168-1. [DOI] [PubMed] [Google Scholar]
- Satoh H, Aoki K, Watanabe SI, Kaneko A. L-type calcium channels in the axon terminal of mouse bipolar cells. Neuroreport. 1998;9:2161–2165. doi: 10.1097/00001756-199807130-00002. [DOI] [PubMed] [Google Scholar]
- Schmitz F. The making of synaptic ribbons: how they are built and what they do. Neuroscientist. 2009;15:611–624. doi: 10.1177/1073858409340253. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7:826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Smith SM, Renden R, von Gersdorff H. Synaptic vesicle endocytosis: fast and slow modes of membrane retrieval. Trends Neurosci. 2008;31:559–568. doi: 10.1016/j.tins.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman J, Zenisek D, Nawy S. Switching between transient and sustained signalling at the rod bipolar-AII amacrine cell synapse of the mouse retina. J Physiol. 2009;587:2443–2455. doi: 10.1113/jphysiol.2008.165241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Vollrath L, Seeliger MW, Jaissle G, Eshkind LG, Leube RE. Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience. 2001;107:127–142. doi: 10.1016/s0306-4522(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Stell WK, Ishida AT, Lightfoot DO. Structural basis for on-and off-center responses in retinal bipolar cells. Science. 1977;198:1269–1271. doi: 10.1126/science.201028. [DOI] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wesseling JF. Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron. 1998;21:415–424. doi: 10.1016/s0896-6273(00)80550-4. [DOI] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. gamma-Aminobutyric acid exerts a local inhibitory action on the axon terminal of bipolar cells: evidence for negative feedback from amacrine cells. Proc Natl Acad Sci U S A. 1987;84:3501–3505. doi: 10.1073/pnas.84.10.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Okada T. Release of endogenous excitatory amino acids from ON-type bipolar cells isolated from the goldfish retina. J Neurosci. 1991;11:2199–2208. doi: 10.1523/JNEUROSCI.11-07-02199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Okada T, Arimura T, Kobayashi K, Piccolino M. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J Neurosci. 1993;13:2898–2909. doi: 10.1523/JNEUROSCI.13-07-02898.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Brandstatter JH. Ribbon synapses of the retina. Cell Tissue Res. 2006;326:339–346. doi: 10.1007/s00441-006-0234-0. [DOI] [PubMed] [Google Scholar]
- Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. J Neurophysiol. 2005;93:1476–1485. doi: 10.1152/jn.00597.2004. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci. 2002;22:10558–10566. doi: 10.1523/JNEUROSCI.22-24-10558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh J, von Gersdorff H. Prolonged reciprical signaling via NMDA and GABA receptors at a retinal synapse. J Neurosci. 2005;25:11412–11423. doi: 10.1523/JNEUROSCI.2203-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994a;367:735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994b;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. J Neurosci. 1996;16:115–122. doi: 10.1523/JNEUROSCI.16-01-00115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J Neurosci. 1997;17:1919–1927. doi: 10.1523/JNEUROSCI.17-06-01919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21(5):1177–1188. doi: 10.1016/s0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–1227. doi: 10.1016/s0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- Von Kriegstein K, Schmitz F, Link E, Sudhof TC. Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur J Neurosci. 1999;11:1335–1348. doi: 10.1046/j.1460-9568.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- von Ruden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- Wahl-Schott C, Baumann L, Cuny H, Eckert C, Griessmeier K, Biel M. Switching off calcium-dependent inactivation in L-type calcium channels by an autoinhibitory domain. Proc Natl Acad Sci U S A. 2006;103:15657–15662. doi: 10.1073/pnas.0604621103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan QF, Vila A, Zhou ZY, Heidelberger R. Synaptic vesicle dynamics in mouse rod bipolar cells. Vis Neurosci. 2008;25:523–533. doi: 10.1017/S0952523808080711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan QF, Zhou ZY, Thakur P, Vila A, Sherry DM, Janz R, Heidelberger R. SV2 Acts via Presynaptic Calcium to Regulate Neurotransmitter Release. Neuron. 2010;66:884–895. doi: 10.1016/j.neuron.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394:384–388. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- Wu LG, Ryan TA, Lagnado L. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J Neurosci. 2007;27:11793–11802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Zhao JW, Yang XL. Expression of voltage-dependent calcium channel subunits in the rat retina. Neurosci Lett. 2002;329:297–300. doi: 10.1016/s0304-3940(02)00688-2. [DOI] [PubMed] [Google Scholar]
- Xu J, McNeil B, Wu W, Nees D, Bai L, Wu L-G. GTP-independent rapid and slow endocytosis at a central synase. Nature Neuroscience. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- Zanazzi G, Matthews G. The molecular architecture of ribbon presynaptic terminals. Mol Neurobiol. 2009;39:130–148. doi: 10.1007/s12035-009-8058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proc Natl Acad Sci U S A. 2008;105:4922–4927. doi: 10.1073/pnas.0709067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Horst NK, Merrifield C, Sterling P, Matthews G. Visualizing synaptic ribbons in the living cell. J Neurosci. 2004;24:9752–9759. doi: 10.1523/JNEUROSCI.2886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. Calcium action potentials in retinal bipolar neurons. Vis Neurosci. 1998;15:69–75. doi: 10.1017/s0952523898151064. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Feldman ME, Almers W. A membrane marker leaves synaptic vesicles in milliseconds after exocytosis in retinal bipolar cells. Neuron. 2002;35:1085–1097. doi: 10.1016/s0896-6273(02)00896-6. [DOI] [PubMed] [Google Scholar]
- Zhou ZY, Wan QF, Thakur P, Heidelberger R. Capacitance measurements in the mouse rod bipolar cell identify a pool of releasable synaptic vesicles. J Neurophysiol. 2006;96:2539–2548. doi: 10.1152/jn.00688.2006. [DOI] [PubMed] [Google Scholar]