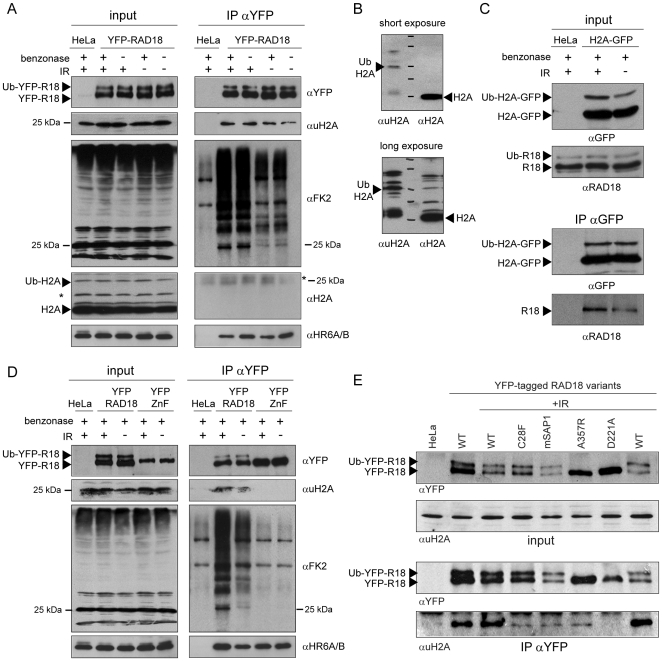
Figure 3. Interaction of RAD18 with ubiquitylated histone H2A via its Zinc finger following IR.
(A) HeLa cells stably expressing YFP-RAD18 were exposed to IR (10 Gy). One hour later, cells were lysed and immunoprecipitation (IP) was performed using an YFP antibody. Subsequently, co-precipitation of ubiquitylated histone H2A (uH2A), conjugated ubiquitin (FK2), histone H2A, and HR6A/B was detected on immunoblots using antibodies as indicated. The expression level of the proteins in the input samples is shown in the left panel. Wild-type HeLa cells were used as a control. The asterisk indicates a nonspecific band (light chain of IgG in IP αYFP, αH2A). (B) Expression level of H2A and ubiquitylated H2A was analysed in HeLa cells using two different antibodies as shown in the figures. (C) Under the same experimental conditions as in (A), HeLa cells stably expressing H2A-GFP were analyzed for the interaction of GFP-H2A with endogenous RAD18. Input samples are shown in the top panels. In the bottom panels (IP αGFP), (co) immunoprecipitation of (ubiquitylated) H2A and RAD18 are shown as indicated. (D) Wild type or Zinc finger mutant (D221A;YFP-ZnF) YFP-RAD18 were expressed in HeLa cells in which endogenous RAD18 was stably downregulated (see Figure S1B and S1C). Under the same experimental condition as in (A), interaction of either YFP-RAD18 or YFP-ZnF with ubiquitylated H2A was analyzed by immunoprecipitation. Subsequently, co-precipitation of uH2A, conjugated ubiquitin, and HR6A/B was detected on immunoblots using antibodies as indicated. The expression level of the proteins in the input samples is shown in the left panel. Wild-type HeLa cells were used as a control. (E) Under the same experimental conditions as in (B) but without benzonase treatment, several RAD18 mutants as indicated were analyzed for their interaction with ubiquitylated H2A.