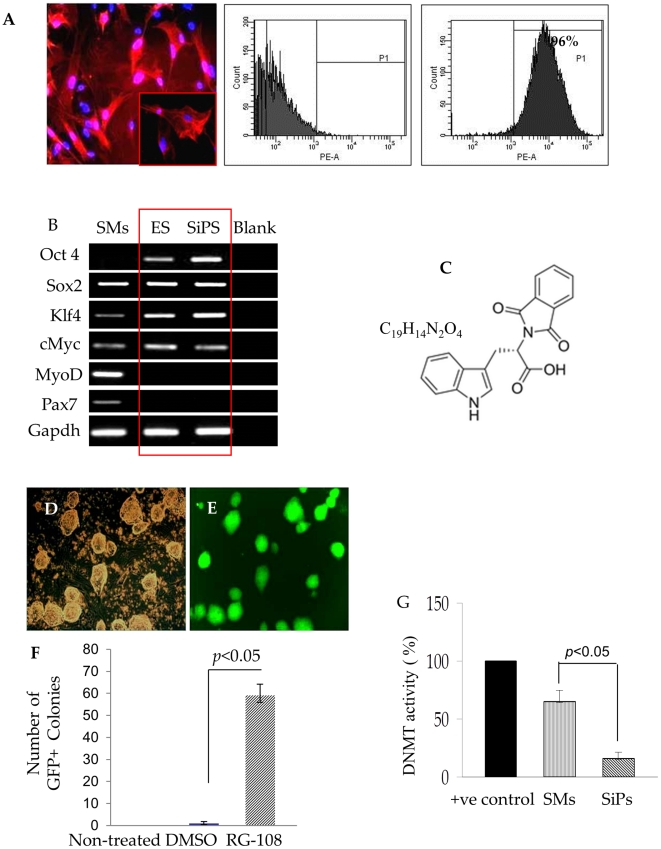
Figure 1. SiPS generation from SMs by single small molecule treatment.
(A) The native SMs cells were stained for desmin expression (red fluorescence) and confirmed by flow cytometry (96%) to show the purity of SMs. The nuclei were stained with DAPI (blue fluorescence). (B) Gene expression profile of various stemness and myogenic markers in SMs, ES cells and SiPS isolates. RT-PCR analyses showing the endogenous expression of myogenic and pluripotency marker genes in SMs and ES cells. SMs showed 3 pluripotency markers (Sox2, Klf4, cMyc) in addition to MyoD and Pax7 in comparison to ES cell expressing all 4 pluripotency markers. RG108 significantly induced Oct3/4 expression in SiPS with simultaneous loss of myogenic markers MyoD and Pax7 after reprogramming. Densitometric quantization of mRNA expression of Oct3/4, Sox2, Klf4, cMyc, MyoD and Pax7 in SMs, ES cells and SiPS are given in Figure S1. (C) Chemical structure of RG108 (D) Phase contrast and (E) fluorescent images of GFP expressing SiPS clones generated by RG108 (500 µM). (F) Graph showing generation of approximately 57–60 GFP+ clones from 50,000 SMs 2–3 weeks after RG108 treatment. The control SMs were either without treatment or treated with DMSO (the solvent for RG108). Similar results were obtained in three independent experiments. (G) DNA methylation analysis showing significant inhibition of DNA methyltransferase activity in SiPS cells in comparison to the untreated SMs. Positive control shown in the graph was provided with the assay kit.