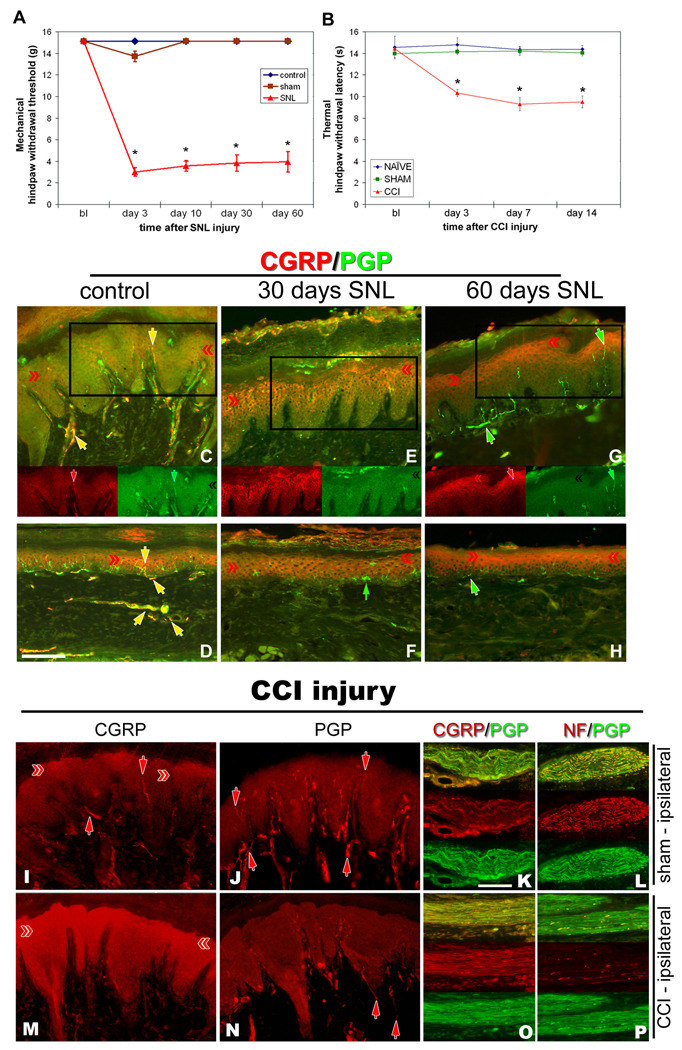
Figure 2. Nerve injury models of neuropathic pain increase CGRP expression in epidermal keratinocytes.
Nocicieptive behaviors were induced in rats by SNL (A) or sciatic CCI (B), and following SNL (C–H) or sciatic CCI (I–P), CGRP-IL increases are evident in hindpaw epidermal keratinocytes (red chevrons), with a reduction in peptidergic cutaneous innervation compared to control skin (yellow and red arrows). A) Graph depicting the extent of chronic neuropathic mechanical allodynia (Von Frey filaments) following SNL, demonstrating persistence out to at least 60 days, at a time corresponding to increased CGRP-IL among the keratinocytes. B) Graph depicting the extent of chronic neuropathic thermal hyperalgesia (Hargreaves test) following CCI, demonstrating persistence out to 14 days, at a time corresponding to increased CGRP-IL among the keratinocytes. C–P) Representative photomicrographs of single or double (merged) label immunocytochemistry for calcitonin-gene related peptide (CGRP, red) or 200kD neurofilament (NF, red) and protein gene product 9.5 (PGP, green). Previous studies have shown that anti-PGP labels all types of innervation, whereas 200kD NF immunolabeling is normally expressed in, and limited to, thick caliber Aβ fibers and thin caliber Aδ fibers. C fibers normally lack NF-IL. CGRP-IL is normally observed in subsets of C and Aδ fibers. C,D) Control, unoperated rats show intense CGRP-IL of axonal innervation (PGP positive, double labeled structures) among the epidermal endings, dermal vasculature endings, and subepidermal and dermal fibers (yellow arrows). Epidermal keratinocyte CGRP-IL (red chevrons) is heterogeneous and moderate among the volar pad crown (C) and flat (D) glabrous hindpaw skin, and remains largely suprabasal. E,F) By 30 days following SNL, a near complete loss of neural innervation is observed (i.e., no PGP nerve labeling; small green arrow in F shows a Langerhans cell, which are known to express PGP), while epidermal keratinocyte CGRP expression appears more extensive throughout suprabasal keratinocytes among the pad crown (E) and flat (F) glabrous hindpaw skin (red chevrons). G, H) By 60 days following SNL, neural innervation has begun to return (PGP axons, green arrows), but largely without a CGRP contingent, whereas the epidermal keratinocyte expression of CGRP remains elevated among pad crown (G) and flat (H) glabrous hindpaw skin (red chevrons). I–L) Sham-operated, non-CCI rats have epidermal keratinocyte expression of CGRP that is heterogeneous and weak among the volar pad crown (I, red chevrons), with intense CGRP immunolabeling of a subset of PGP-positive axonal innervation (I, red arrows; J, PGP positive, red arrows) among the epidermal endings (downward red arrows), and upper dermal fibers (upward red arrows), and within dermal nerves (K, red panel). NF content within the dermal nerves was extensively distributed among mostly the larger caliber axons and some small caliber axons (L, red panel). M–P) CCI rats showed dramatically elevated expression of CGRP among the suprabasal keratinocytes (M, red chevrons), coincident with depleted CGRP-IL epidermal endings and dermal nerve fibers, among an overall depleted innervation (N, PGP positive, red arrows). Interestingly, CGRP content appears to be increased within deep dermal nerves (O, red panel), but was mostly distributed among vacuolated cells affiliated with the larger caliber axons. NF-IL is severely depleted among the large caliber axons within the deep nerves, indicating that these axons may be deteriorating and that the vacuolated cells may be reactive Schwann cells (P, red panel). Magnification bar (D) =50µm for C–H, and I,J,M,N; (K)=25µm for K,L,O,P.