Table 1.
Directed heterodimer synthesis.
| Entry | Unsymmetrical Sulfamidea | Diazeneb | Heterodimeric Productc |
|---|---|---|---|
| 1 | 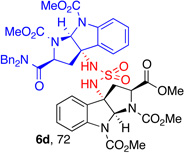 |
5d, (80) | 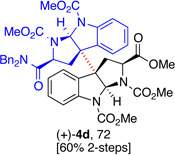 |
| 2 | 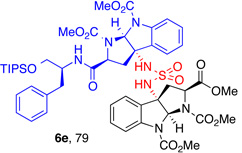 |
5e, (88) | 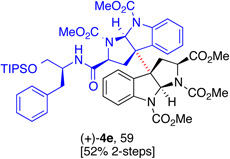 |
| 3 | 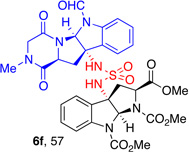 |
5f, (83) | 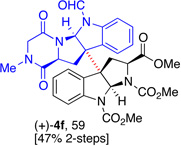 |
| 4 | 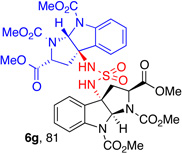 |
5g, (99) | 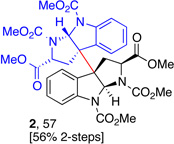 |
| 5 | 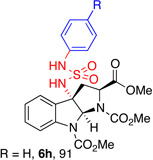 |
5h, (91)d | 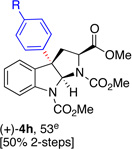 |
| 6 | R = CF3, 6i, 57 | 5i, (99) | (+)-4i, 61 [60% 2-steps] |
Mixed sulfamide synthesis: 7, 13, DMAP, Et3N, CH2Cl2, 0 → 23 °C. Isolated % yield of 6 after chromatography.
Diazene synthesis: BEMP, NCS, THF, 23 °C. Crude % yield of sensitive diazene 5 in parentheses.
Heterodimer synthesis: t-BuOH, hν >280 nm, 23 °C, 5 h. Isolated % yield of 4 after chromatography. Yield of 4 from 6 in brackets.
DBU, NCS, MeOH, 0 → 23 °C.
hν 300 nm, 12 h.
