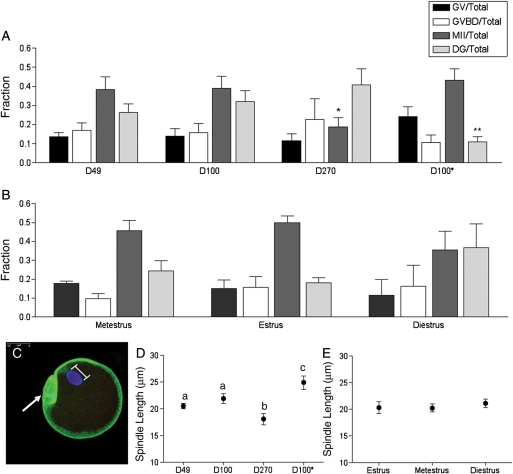
Figure 4.
In vitro maturation results and spindle morphology in the oocyte following in vitro follicle growth. On Day 9 of culture, oocytes were in vitro matured by hormonal stimulation, and light microscopy was used to assess meiotic stage (GV, germinal vesicle intact; GVBD, germinal vesicle breakdown; MII, metaphase II-arrested; DG, degenerate). Meiotic progression is shown for oocytes obtained from (A) follicles isolated from mice of different ages (D49, D100 and D270) and weights (D100 and D100*) as well as (B) those isolated from D49 mice at different stages of the estrus cycle. Meiotic progression was independently compared among follicles isolated from mice of different ages, weights or estrous cycle stages. The fraction of oocytes that reached MII was significantly lower in the D270 cohort compared with the D49 and D100 cohorts (denoted by*). The fraction of degeneration observed in the D100* cohort was significantly less than that observed in the D100 cohort (denoted by**). Following in vitro maturation, MII-arrested oocytes were fixed and immunostained to visualize cortical actin (green), the spindle (blue) and chromosomes (red) by confocal microscopy. A minimum of 15 oocytes per cohort was analyzed, and a representative oocyte is shown in (C) (arrow marks the polar body). The spindle length for each oocyte was determined by a pole-pole measurement as illustrated in (C). The spindle length in oocytes from follicles isolated from (D) mice of different ages and weights and from (E) D49 mice at different stages of the estrous cycle are shown. The spindle length was greater in D49 and D100 cohorts compared with the D270 cohort (P<0.05), as well as in the D100* cohort compared with the D100 cohort. There were no statistically significant differences in spindle length based on cycle stage.