FIGURE 1.
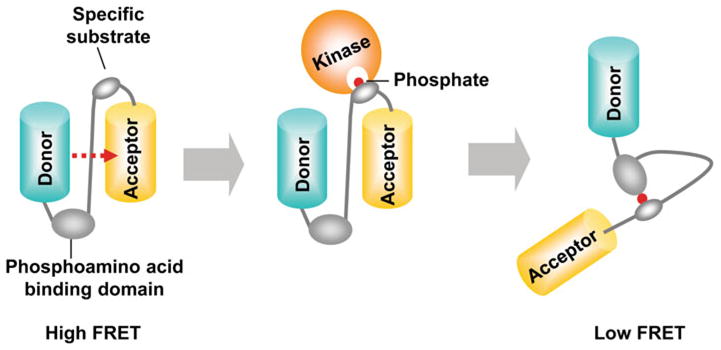
General design of a tyrosine kinase biosensor based on FRET. A typical kinase biosensor based on FRET is composed of a specific substrate peptide and its binding domain between a donor and an acceptor FPs. When the specific substrate in the biosensor is phosphorylated by active kinase, it can bind to the nearby binding domain, e.g. SH2 domain. This intramolecular interaction can cause the conformation change of the biosensor, which results in the FRET change.
