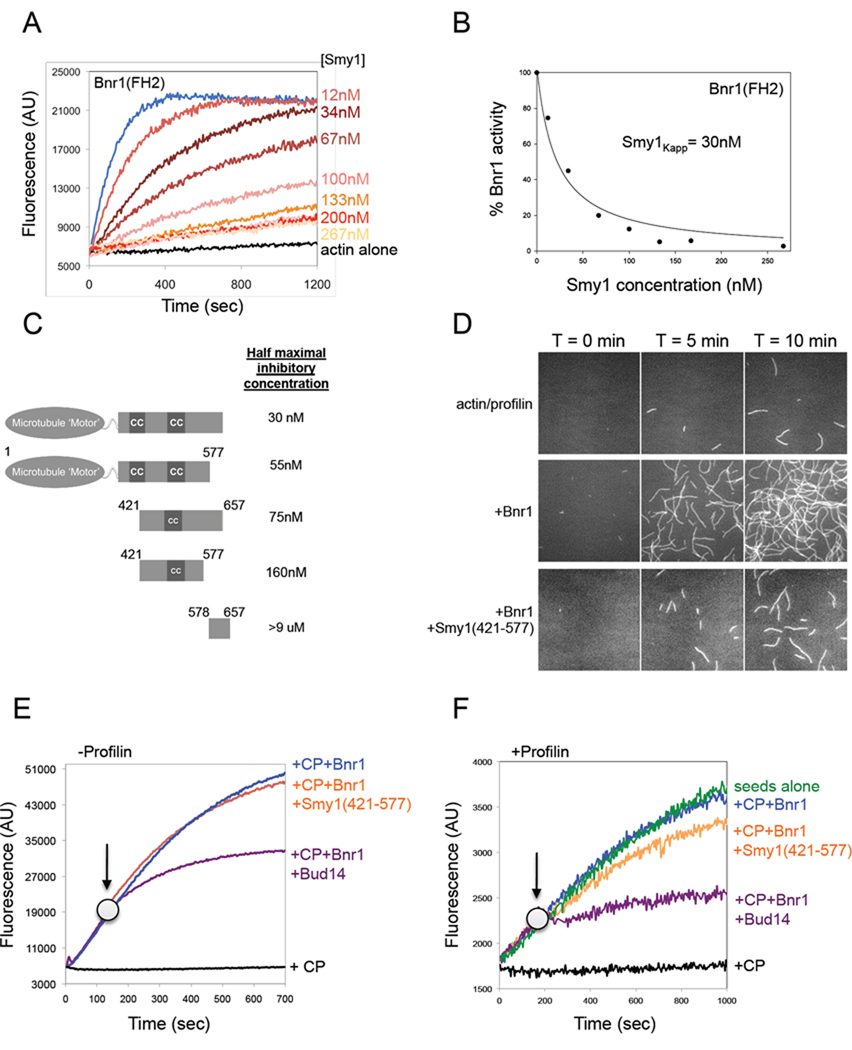
Figure 1. Smy1 inhibits Bnr1 (FH2) actin nucleation activity, but not processive capping.
(A) Monomeric actin (2µM, 5% pyrene labeled) was polymerized in the presence of 5nM Bnr1 (FH2) and the indicated concentrations of Smy1. (B) Concentration-dependent inhibitory effects of Smy1 on Bnr1. Percent Bnr1 activity was determined by dividing the slope of the actin polymerization curve in the presence of Smy1 by the slope of the curve in the absence of Smy1. (C) Schematic of purified full length and truncated Smy1 polypeptides. Each polypeptide was compared at a range of concentrations for its inhibitory effects on Bnr1 (FH1-FH2-C) in pyrene-actin assays (see Supplementary Figure 1, H–J) to determine concentration required for half-maximal inhibition. (D) Time-lapse TIRF microscopy on assembly of 1µM actin and 3µM profilin in the presence of control buffer, 1nM Bnr1 (FH1-FH2-C) and/or 500nM Smy1 (421–577). (E) Effects of Smy1 and Bud14 on Bnr1-capped actin filament barbed end growth in the presence of capping protein (CP). At time zero, monomeric actin (0.5µM, 10% pyrene labeled) was added to mechanically sheered unlabelled F-actin seeds (0.3µM) in the presence of 0.7nM Bnr1 (FH1-FH2-C) and 500nM S. cerevisiae CP. At the indicated time point (arrow) either 500nM Bud14 (179–472) or 500nM Smy1 (421–577) was added. (F) Effects of Smy1 and Bud14 on Bnr1-capped actin filament barbed end growth in the presence of capping protein (CP) and profilin. Same as in E, but reactions contained 3µM profilin.