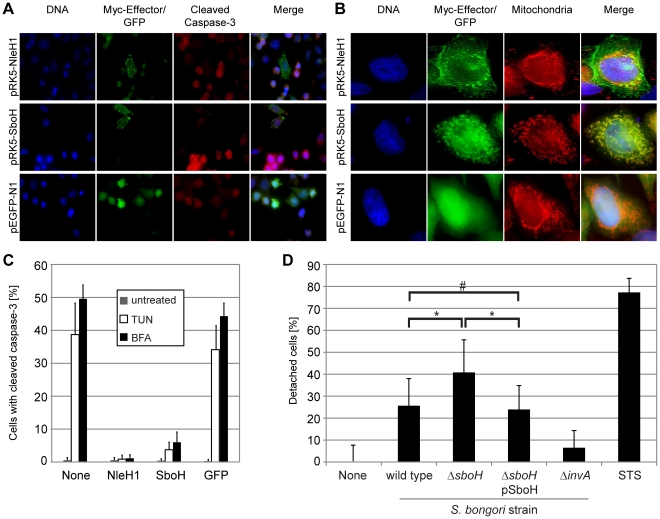
Figure 5. The chimeric effector SboH combines features of NleH1 and SopA and reduces host cell detachment during infection.
A–C. To compare the phenotypes of SboH and NleH1 HeLa cells were transfected with pRK5-nleH1, pRK5-sboH or the control plasmid pEGFP-N1 and processed. A. SboH inhibits tunicamycin (TUN) and brefeldin A (BFA) induced caspase-3 activation. Immunofluorescence analysis of transfected HeLa cells treated with 5 µg/mL TUN or 10 µg/mL BFA for 18 h and stained for DNA (blue), Myc-tagged effectors (green) and activated caspase-3 (red). GFP, but not SboH and NleH1 expressing cells frequently stain positive for activated caspase-3. (Images are representative of both TUN and BFA treated cells). Bar = 10 µm. B. The level of inhibition of caspase-3 activation by SboH or NleH1 was quantified by immunofluorescence counting of transfected cells. C. SboH and NleH1 are targeted to different subcellular locations. Transfected HeLa cells were stained for DNA (blue), Myc-tagged effector (green) and mitochondria (red) and analysed by immunofluorescence microscopy. SboH nearly exclusively co-localises with the mitochondrial marker MitoTracker, whereas NleH1 shows plasma membrane and perinuclear localisation. Scale bar = 10 µm. D. Cells infected with S. bongori ΔsboH show increased cell detachment. Quantification of HeLa cells lost following 5 h infection with S. bongori wild type, ΔinvA mutant, ΔsboH mutant and ΔsboH pSboH complemented strain. Staurosporine (STS) was used as a positive control to induce cell detachment. The one-way ANOVA Test using Bonferroni correction was used on data from five independent experiments and showed that the differences between S. bongori wild type and ΔsboH, as well as between S. bongori ΔsboH and the complemented strain are significant (* p-value<0.001). There was no significant difference between S. bongori wild type and the complemented strain (# p-value >0.05).