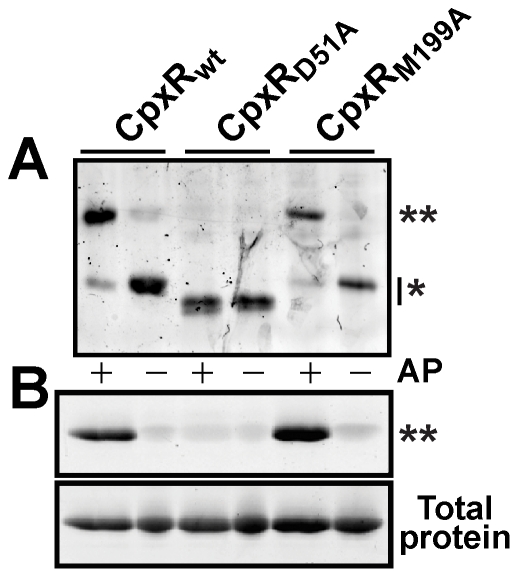
Figure 2. Phosphorylation of the CpxR residue Asp51.
Wild type (CpxRwt::His6) and defined variants with the amino acid substitutions Asp51Ala (CpxRD51A::His6) and Met199Ala (CpxRM199A::His6) were purified by metal affinity chromatography. Purified proteins (1.25 µM) were incubated in the presence of 50 mM acetyl∼P (AP) (+). In A, samples were fractionated by a Manganese(II)-Phos-tag™ 12.5% acrylamide gel and then stained by the SYPRO® Ruby staining method. In B, samples were separated in a conventional SDS 12% polyacrylamide gel. Phosphorylated protein (upper panel) was specifically detected by the Pro-Q® Diamond phosphoprotein gel stain technique, while total protein (lower panel) was visualized by the SYPRO® Ruby staining method. Phosphorylated CpxR is indicated by a double asterisk and unphosphorylated CpxR by a single asterisk. The reason for the enhanced mobility of CpxRD51A::His6 when fractionated by Manganese(II)-Phos-tag™ acrylamide (Panel A) is uncertain.