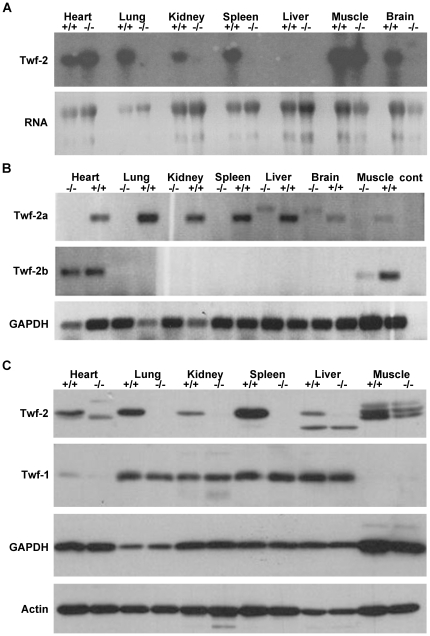
Figure 2. Northern blot, RT-PCR, and Western blot analysis of twinfilin-2a knockout mice.
Total RNAs and proteins were isolated from 3-month-old wild-type and twinfilin-2a knockout littermates. (A) Northern blot analysis of the total RNA of wild type (+/+) and knockout (−/−) mice hybridized with a twinfilin-2 cDNA probe. Twinfilin-2 RNA is absent from all mutant tissues, except in heart and skeletal muscles. The remaining twinfilin-2 mRNA encodes for the twinfilin-2b isoform. Equal loading between the wild-type and knockout RNAs is demonstrated in the lower panel. (B) Agarose gel electrophoresis of products from RT-PCR of total tissue RNA of wild type (+/+) and knockout (−/−) mice. Twinfilin-2a mRNA is absent from all mutant tissues, whereas twinfilin-2b expression remains in heart and skeletal muscles of twinfilin-2a knockout mice. Lower panel shows control reactions carried out by using GAPDH-specific primers. The slower mobility band detected in liver and brain of knockout mice was isolated and sequenced and turned out to be an unspecific product of an unrelated protein. (C) Western blot of tissue homogenates of wild type (+/+) and knockout (−/−) mice probed with twinfilin-2 (Twf-2) or twinfilin-1 (Twf-1) specific polyclonal antibodies. Twinfilin-2 protein was absent from all other mouse tissues tested except heart and skeletal muscles. Please note that the twinfilin-2 antibody used here does not distinguish between twinfilin-2a and twinfilin-2b isoforms. GAPDH and actin are shown as loading controls.