Figure 1.
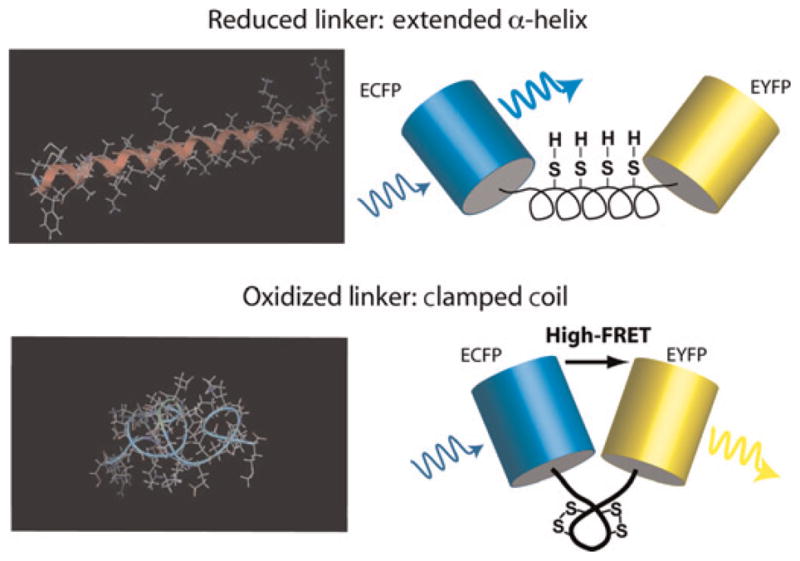
Schematic of the redox biosensor. Minimum energy configurations for dispersed cysteine residues are shown on the left – with (bottom) and without (top) locking the disulfide bonds – and were calculated in MOE (Molecular Operating Environment; Chemical Computing Group, Montreal, Canada). On the right, cartoons of the final redox-sensitive biosensor design (CY-RL7) are shown to illustrate the conformational change that leads to enhanced Förster resonance energy transfer (FRET) in the oxidized ‘clamped coil’ state compared with the reduced α-helical low-FRET state
