Figure 4.
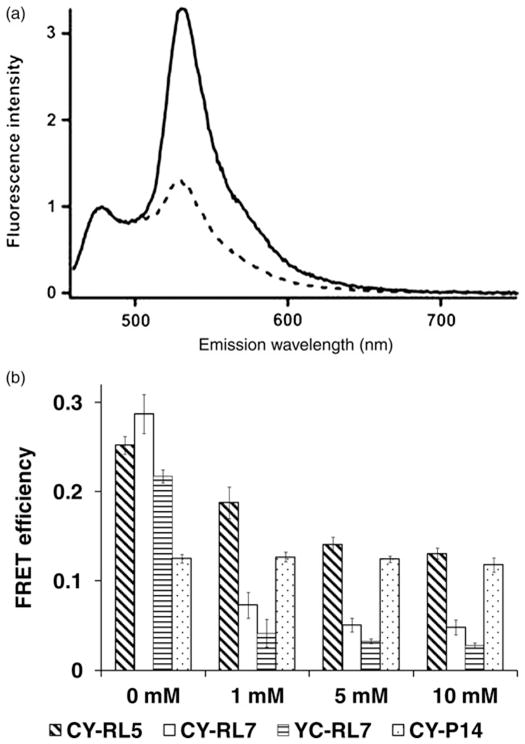
(a) Fluorescence emission spectra of the CY-RL7 sensor at 20°C, where the solid line corresponds to untreated sensor and the dashed line to the protein pretreated with 10 mmol/L DTT. Purified protein was excited at 440 nm and spectra were normalized to the intensity of the ECFP peak (475 nm). (b) FRET response to DTT treatment for purified constructs CY-RL5, CY-RL7 and YC-RL7. Values given are the average of three experiments ± SD. Differences of calculated FRET efficiencies between untreated (0 mmol/L DTT) and DTT-treated sensors CY-RL5, CY-RL7 and YC-RL7 were statistically significant (P < 0.05). Significant differences were not observed for the polyproline CY-P14 construct (snow bars; P > 0.05). DTT, dithiothreitol; FRET, Förster resonance energy transfer
