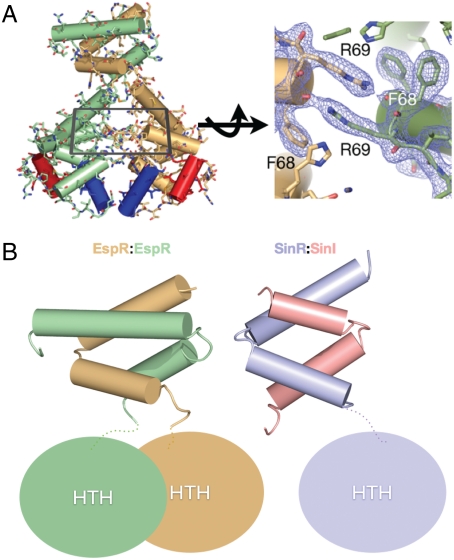
Fig. 2.
Dimerization interfaces of EspR. (A) The interactions between the HTH domains are primarily electrostatic in nature with a set of symmetrically stacked arginines and phenylalanines at the center of the interface. (B) Comparison of the CTD of the EspR dimer (gold and green) with the SinR:SinI heterodimer (purple and pink). In both structures, the CTDs form a four-helix bundle and the dimer interface between them is entirely hydrophobic.