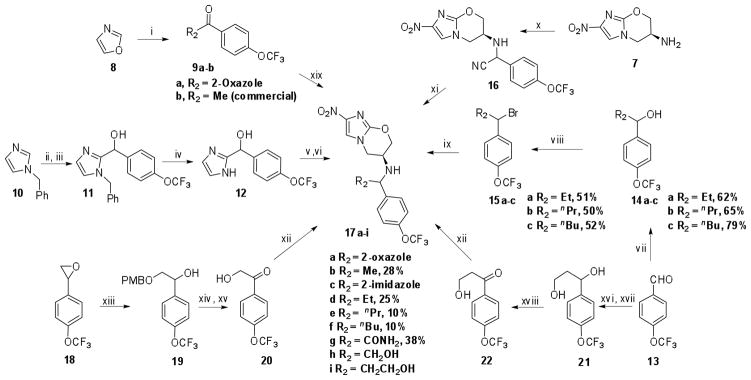
Scheme 2.
Reaction conditions: n-BuLi, ZnCl2, CuI, THF, −78 °C – rt, 1.5 h, then 4-trifluoromethoxybenzoyl- chloride, rt, 1 h, 40%; ii) diisopropylcarbamyl chloride, DIPEA, 4-trifluoromethoxybenzaldehyde, CH3CN, reflux, 19 h, 73%; iii) 50 % TFA in water, THF, reflux, 15 h, 82%; iv) H2, Pd/C, MeOH, 1 atm, 81%; v) MsCl, Et3N, CH2Cl2, rt, 1 h; vi) 7, NaH, THF, rt, 40 h, 15%.; vii) RMgBr, THF, 0 °C – rt when R = Et and nPr; nBuLi, THF, −78 °C – rt when R = nBu; viii) PBr3, ether, 0 °C – rt; ix) 7, K2CO3, DMF, KI, 90 °C.; x) 4-trifluoromethoxybenzaldehyde, neat, 100 °C, 5 min. then TMSCN, 100 °C, 30 min, 50%; xi) EtOH/HCl, −10 °C, 38%; xii) 7, NaCNBH3, AcOH, EtOH, 5%; xiii) 4-methoxybenzylalcohol, KOtBu, 60 °C, 2 h, 34%; xiv) PDC, CH2Cl2, rt, 24 h, 62%; xv) TBDMSOTf, CH2Cl2, rt, 5 min, 86%; xvi) ethylbromoacetate, Zn, CH2Cl2, rt, 3 h; xvii) LiAlH4, THF, 0 °C - rt, 2 h, 25% over two steps; xviii) MnO2, CH2Cl2, rt, 6 h, 50%; xix) 7, Ti(iOPr)4, AcOH, NaBH3CN, 9%.