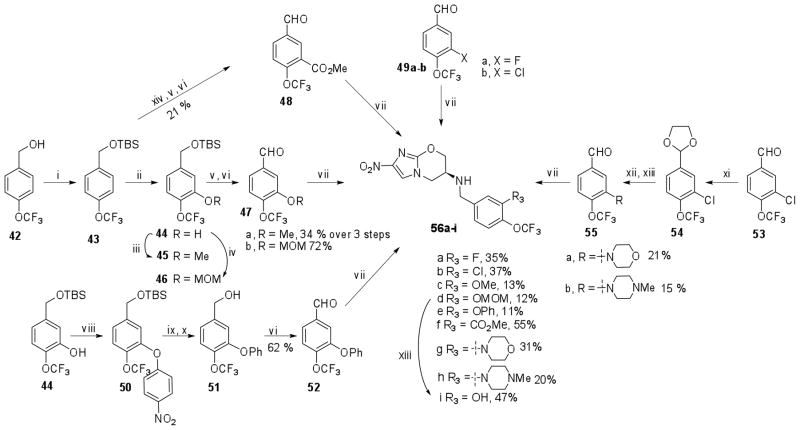
Scheme 5.
Reaction conditions: i) TBSCl, imidazole, CH2Cl2, rt, 60%; ii) s-BuLi, TMEDA, THF, −78 °C, 1 h, then FB(OMe)2, −78 °C, 30 min followed by alk, H2O2, 30 min, 28%; iii) K2CO3, MeI, DMF, 70 °C; iv) MOMCl, DIPEA, DMF, rt, 16 h, 82 %; v) TBAF, THF, rt, 1.5 h; vi) PCC, CH2Cl2, rt, 1 h; vii) 7, NaCNBH3, AcOH, DMF; viii) 4-fluoronitrobenzene, NaH, DMF, 100 °C, 2 h, 30 %; ix) Fe/NH4Cl, EtOAc-water, reflux, 1.5 h; x) NaNO2, H3PO2, 6N HCl, 50 °C, 1 h, 52 % over two steps; xi) ethylene glycol, p-TSA, benzene, 80 °C, 8 h, 72 %; xii) Pd(OAc)2, Cs2CO3, Xantphos, dioxane, amine, 90 °C, 8 h; xiii) THF, 6 N HCl, 30 min., rt; xiv) sec-BuLi, MeOCOCl, THF, −78 °C – rt, 3 h.