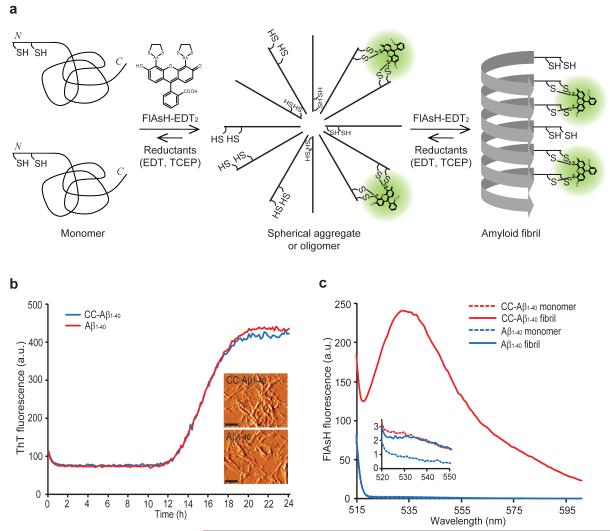
Figure 1.
Detection of Cys-Cys-Aβ1-40 fibrils using FlAsH-EDT2. (a) A model wherein Aβ1-40 with two consecutive cysteines at its N terminus (Cys-Cys-Aβ1-40, or CC- Aβ1-40) first forms spherical aggregates and then fibrils, creating in trans tetra-cysteine binding sites for FlAsH binding and fluorescence. EDT = 1,2-ethanedithiol, TCEP = tris(2-carboxyethyl)phosphine (b) Aggregation time courses of initially monomeric Aβ1-40 or CC-Aβ1-40 monitored by ThT fluorescence. The inset shows AFM images of CC-Aβ1-40 fibrils and Aβ1-40 fibrils. Scale bar = 250 nm. AFM images are shown in amplitude mode. (c) Monomeric CC-Aβ1-40 (10 μM) or fibrillar CC-Aβ1-40 was incubated with FlAsH-EDT2 (100 μM) for 1 h at 25 °C. After removing unbound FlAsH-EDT2, FlAsH fluorescence was measured (ex. 508 nm). The inset shows non-detectable binding of FlAsH to monomeric CC-Aβ1-40, monomeric Aβ1-40, and fibrillar Aβ1-40. Representative figures of at least three different experiments are shown.