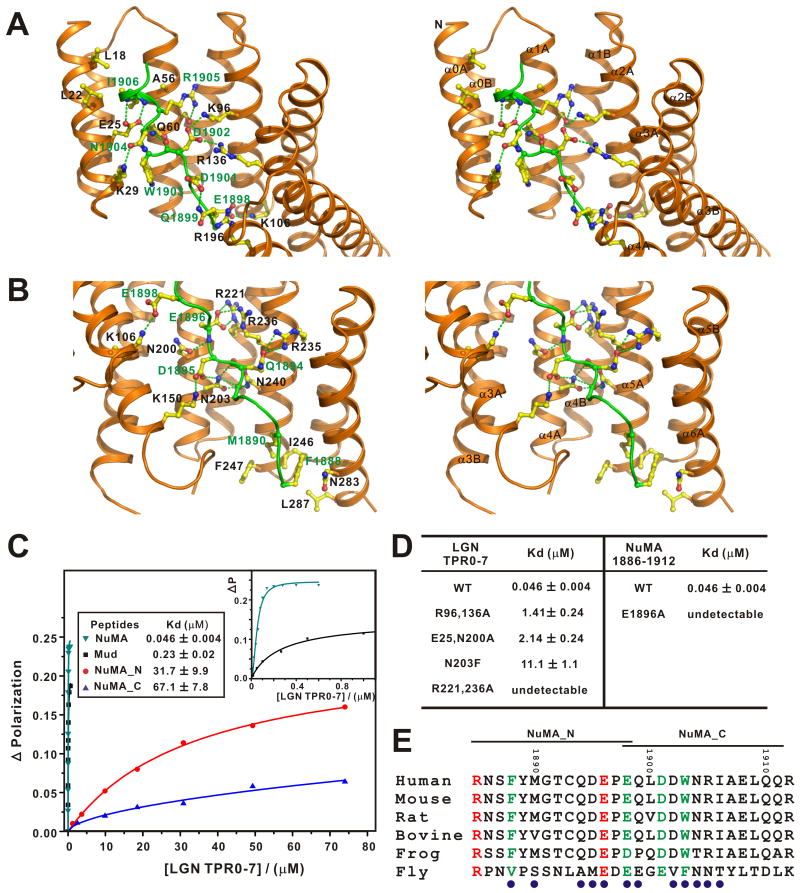
Figure 2. The interaction interface of the LGN/NuMA complex.
The LGN/NuMA interface is divided into two parts corresponding to LGN TPR0-3/NuMA_C (A) and LGN TPR4-7/NuMA_N (B). The interaction details between LGN and NuMA in the two parts are shown in stereo-view. The sidechains of the residues involved in the inter-domain interactions are drawn in the stick model. Charge-charge and hydrogen bonding interactions are highlighted by dashed lines in green. (C) Fluorescence polarization-based measurement of the binding affinities of LGN TPR0-7 to various NuMA and Mud peptides (sequences shown in panel E). The insert shows the expanded view of the binding curves of the NuMA and Mud peptides to LGN TPR0-7. (D) Summary of the bindings of LGN TPR0-7 and its mutants with the NuMA peptides. (E) Sequence alignment of the NuMA peptide showing that the residues involved in making contact with LGN are evolutionary conserved. The residues involved in the LGN interaction are indicated with blue circles.