Abstract
Background
Resistance to the antimalarial drug sulfadoxine-pyrimethamine (SP) emerged in Plasmodium falciparum from Asia in the 1960s and subsequently spread to Africa. In Tanzania, SP use as a national policy began in 1983 as a second line to chloroquine (CQ) for the treatment of uncomplicated malaria, until August 2001 when it was approved to replace CQ as a national first line.
Objective
The present study assesses the frequency of resistant dhfr and dhps alleles in Morogoro-Mvomero district in south eastern Tanzania and contrast their rate of change during 17 years of SP second line use against five years of SP first line use.
Methodology
Cross sectional surveys of asymptomatic infections were carried out at the end of rainy season during July–September of 2000, when SP was the national second line (CQ was the first line) and 2006 when SP was the national first line antimalarial treatment. Genetic analysis of SP resistance genes was carried out on 1,044 asymptomatic infections and the effect of the two policies on SP evolution compared.
Results
The frequency of the most resistant allele, the double dhps-triple dhfr mutant genotype, increased by only 1% during 17 years of SP second line use, but there was a dramatic increase by 45% during five years of SP first line use.
Conclusion
We conclude that National policy change from second line to first line SP, brought about an immediate shift in treatment practice and this in turn had a highly significant impact on drug pressure. The use of SP in specific programs only such as intermittent preventive treatment of infants (IPTi) and intermittent preventive treatment of pregnant women (IPTp) will most likely reduce substantially SP selection pressure and the SP resistance alleles alike.
Keywords: Plasmodium falciparum infection, Sulfadoxine/Pyrimethamine resistance, Polymerase Chain Reaction, Sequence Specific Oligonucleotide Probing
Introduction
1The United Republic of Tanzania has a population of more than 36 million, all of whom are at risk of malaria. However, endemicity and risk of transmission varies and have been mapped by the MARA collaboration1. This GIS-based analysis revealed that 75% of the population is subject to stable perennial or stable seasonal malaria transmission; 8% to unstable highly seasonal transmission; and 17% to no malaria transmission in the average year, but still at risk of epidemic malaria.
The Morogoro-Mvomero population is among the 75% group which is subject to stable perennial transmission with some seasonal variations, where peak transmission occur during end of rainy season in July to September of each year. Control of malaria largely depends on early detection and treatment with efficacious drugs. However, Malaria parasites have become increasingly drug-resistant, and resistance has spread geographically2, 3, 4, 5, 6, 7, 8, 9. A survey of SP efficacy prior to national policy change from SP to Artemether+Lumefantrine (ALU) showed a marked regional variability within Tanzania with clinical efficacy ranging between 33% in Ipinda, 43% in Mlimba to 49.3% in Mkuranga10, 11. Although SP was replaced by combination of Artemether+Lumefantrine (ALU) as a national first line treatment of uncomplicated malaria in 2006, it remains the only option for intermittent preventive treatment of malaria during pregnancy (IPTp) and an important drug in the intermittent preventive treatment of infant (IPTi). Currently, trials using SP for IPTi indicate variable protective efficacies (PEs) against clinical malaria ranging from 20.1 – 58.6% 12, 13. A number of factors including the intensity of transmission, the pattern of antimalarial resistance and the use of additional control measures such as insecticide treated nets (ITN) have been proposed to contribute to the observed variations in the PEs14.
Treatment of malaria in Tanzania is typically guided by official recommendations from the Ministry of Health and Social Welfare (MOHSW) regarding drugs of choice for various situations. “First-line” treatment refers to the drug officially recommended as the drug of first choice for the treatment of uncomplicated malaria. “Second-line” treatment refers to the drug officially recommended as an alternative primarily to be used for treatment of patients in whom the first-line treatment failed to clear the infection and other select patients (such as those who are hypersensitive to the first-line treatment). “Third-line” treatment typically refers to the drug recommended for severely ill patients (a rescue drug). In practice, few treatment failures are recognized and patients are often moved directly from first to third-line treatment, consequently, little second-line drug is used compared to the first-line drug. SP replaced CQ as the recommended first- line treatment on the Tanzanian mainland in August 2001, after 18 years of its use as a second-line treatment since 1983.
Evidence of CQ use in Malawi indicates, 12 years after its withdrawal for use in the country, CQ-sensitive parasites re-emerged permitting its reintroduction for malaria treatment15. The Malawian CQ experience has stimulated interest among researchers and they are now exceedingly keen to learn whether a similar trend could be observed in SP following its replacement with ACT. Documentation of the prevalence of SP resistance markers (dhfr and dhps alleles) at the time of its withdrawal in Tanzania in 2006 is necessary to provide a baseline data for the monitoring of temporal trends of these alleles in the population thereafter.
We have examined the genetic change at dhfr and dhps loci under second and first line SP use in Morogoro-Mvomero districts. We collected malaria parasites from residents of randomly selected households in two cross sectional surveys. The first survey occurred during July–September 2000, when CQ was first line treatment and SP was second line. The second survey was conducted in July – September 2006, five years after adoption of a new national policy change from CQ to SP first line treatment of uncomplicated malaria which was also the time of SP replacement with ALU combination therapy as the national first line antimalarial therapy. Infections identified at community level were mainly asymptomatic. This method of sampling we believe is less biased compared to the health facility approach where by definition all infections are symptomatic and many are usually pre-treated cases.
Methods
Study area, subjects and samples
Community surveys were conducted during July, August and September of 2000 and 2006 in initially a one district of Morogoro (Population = 570,422) which was partitioned into Morogoro (Population = 290,316) and Mvomero (Population = 280,106) in 2002. The households that were sampled in 2000 survey were maintained in the 2006, this time belonging to two administratively separate districts of Morogoro and Mvomero but broadly similar in all other aspects and hence referred in this report as one district of Morogoro-Mvomero. The surveys were part of large combination therapy pilot implementation programme in Tanzania, the Interdisciplinary Monitoring Programme for Antimalarial Combination Therapy (IMPACT-TZ). A total of 6,301 adults and children belonging to randomly selected households participated in the study. A finger-prick blood sample for blood slide and filter paper bloodspot were collected from each individual in the household. The filter paper bloodspots were air-dried and stored at room temperature in self-sealing plastic bags with desiccant and stored on dry area at room temperature. All blood slide samples were screened by microscopy for P. falciparum. Bloodspots from microscopically positive subjects were selected and preserved at room temperature for molecular genotyping.
Ethics
Scientific and ethical clearance was granted from the Medical Research Council of the National Institute for Medical Research in Tanzania, the Centers for Disease Control and Prevention, USA, and the London School of Hygiene and Tropical Medicine. Consent was obtained from all individuals or their guardians before collection of samples.
DNA extraction
The DNA was extracted from bloodspots dried on filter papers. A section of the dried blood spot filter paper was excised using a sterile blade or scissors, and soaked in 1 ml of 0.5% saponin in 1x phosphate buffered saline (PBS) overnight in a 96-deepwell plate. The segment was then washed twice in 1 ml of 1x PBS and was finally boiled for 8 min in 150ìl solution containing 100ìl PCR quality water and 50ìl of 20% chelex suspension (pH 9.5).
PCR amplification
Nested PCR was used to amplify a 594 base pair (bp) fragment of dhfr and a 711 bp fragment of dhps each containing the sequences where mutations are found. Primer sequences and PCR reaction conditions were previously described in Pearce et al16. PCR was performed in 96 well plates with 25ìl PCR reaction volumes containing final concentrations of 0.25ìM oligonucleotide primers, 2mM MgCl2, 250ìM each deoxyribonucleotide triphosphate (dNTPs), and 1x Taq polymerase. 1ìl of DNA template was used in the outer (primary) PCR reaction mixture for dhfr and dhps amplifications. For the inner (secondary) dhps reactions 1 ìl of the outer PCR product was used. The outer dhfr PCR products were diluted three fold before a 1 ìl was introduced into the inner PCR reaction mixtures.
Molecular genotyping of point mutations by Sequence Specific Oligonucleotide Probing (SSOP)
The PCR amplified sequences from the coding regions of dhfr and dhps genes were fixed onto membranes and probed with SSOP designed to detect each of the single base pair substitutions at all positions summarized in Table 1. The SSOP method has advantages for high throughput, while retaining sensitivity and specificity equivalent to those of other methods used for detection of dhfr and dhps SNPs. The amplified PCR products were screened for dhfr and dhps sequence variants at 10 loci where single nucleotide polymorphisms (SNPs) are known. The sequence changes (and the amino acid substitutions they code for) are summarised in Table 1.
Table 1.
The nucleotide and amino acid substitutions at (a) dhfr and (b) dhps genes screened for by PCR-SSOP
| a) dhfr | |||||
| Codon | 50 | 51 | 59 | 108 | 164 |
| Wild type | Cys (C) | Asn (N) | Cys (C) | Ser (S) | Ile (I) |
| TGT | AAT | TGT | AGC | ATA | |
| AAC | |||||
| Mutant* | Arg (R) | Ile (I) | Arg (R) | Asn (N) | Leu (L) |
| CGT | ATT | CGT | AAC | TTA | |
| Thr (T) | |||||
| ACC | |||||
| b) dhps | |||||
| Codon | 436 | 437 | 540 | 581 | 613 |
| Wild type | Ser (S) | Ala (A) | Lys (K) | Ala (A) | Ala (A) |
| TCT | GCT | AAA | GCG | GCC | |
| Mutant* | Phe (F) | Gly (G) | Glu (E) | Gly (G) | Ser (S) |
| TTT | GGT | GAA | GGG | TCC | |
| Ala (A) | Thr (T) | ||||
| GCT | ACC | ||||
| Cys (C) | |||||
| TGT | |||||
indicates altered sequences and the resultant amino acid residue at a given SNP locus
PCR products were spotted in a 12 by 8-grid and cross linked onto nylon membranes and probed for sequence polymorphisms by hybridisation to specific oligonucleotide probes described previously16. For analysis of samples collected in 2000, the visualization of hybridised digoxygenin labelled probes on membranes was performed by the alkaline phosphatase-catalysed breakdown of the CSPD substrate (Roche Boehringer Mannheim, Mannheim, Germany) and visualised by exposure on Hyperfilm-ECL (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom), according to Boehringer Mannheim recommendations and previously described by Conway et al17. For analysis of samples collected in 2006 the probed blots were visualised using ECF substrate and detection using a phosphoimager (STORM). Inspection of autoradiographic films was carried out by light box illumination, while the phosphoimager output was recorded through viewing of digitally captured images of chemiflourescent signal. The change in the method by which probe hybridisation signal was visualised did not affect the results in any way since the probes and hybridisation conditions were unchanged.
The stringency and specificity of the hybridisation process was confirmed by inspection of a series of 4 controls with a known single genotype variant sequence. All blots with non-specifically bound probes were stripped and reprobed. A SNP was considered to be present in the PCR product when the intensity of signal was higher than that of the background. The blots were scored independently by two people.
In our analysis we aimed to establish the relative abundance of different point mutation haplotypes at dhfr and dhps. Since bloodstage P. falciparum is haploid, this is very straightforward when an infection consists of a single genotype because only one form of sequence at every SNP locus is seen. When infections are composed of multiple genotypes a mixture of different sequence variants occur making the inference of point mutation haplotypes within that infection more difficult.
The presence, absence, and relative abundance of hybridisation signal for every probe were recorded at each locus. A sample was considered to have a single haplotype when only one sequence variant was found at each locus. Blood samples were categorised as having a single, a majority or a mixture of sequence at every SNP locus. Majority and mixed genotype infections were differentiated according to the relative intensity of signal. To determine the relative abundance of different point mutation haplotypes in the parasite population, one haplotype only was counted from each infection and those mixed infections where haplotypes could not be resolved were omitted from the calculation of haplotype frequencies. Hence, frequency data is based upon a subset of isolates which were unmixed or had a predominating majority haplotype. A breakdown of the proportions of isolates which successfully PCR amplified and which were genotyped as single, majority or mixed haplotype infections is given in Table 2.
Table 2.
Proportion of samples that were P. falciparum positive, PCR amplified dhfr or dhps, and single/majority dhfr or dhps in Morogoro-Mvomero
| Item\Year | 2000 | 2006 |
| Survey population | 1681 | 4620 |
| P. falciparum positive | 347 | 697 |
| (21%) | (15%) | |
| PCR amplified dhfr | 246 | 528 |
| PCR amplified dhps | 244 | 524 |
| Single or majority dhfr | 180 | 471 |
| Single or majority dhps | 165 | 430 |
| Single or majority dhfr+dhps | 98 | 380 |
Statistical Analysis
Statistical comparison of allele frequencies at dhfr and dhps in the various sites was carried out using chi squared analysis in STATA version 9.2. The calculation of binomial exact 95% confidence intervals was carried out using STATA version 9.2. Linkage disequilibrium analysis was performed using Arlequin software18.
Role of the funding source
This study was funded through an interagency agreement between the United States Agency for International Development (USAID) and CDC and a cooperative agreement between CDC and the Ifakara Health Research and Development Centre (IHRDC). USAID did not participate in the design, collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit for publication.
Results
Of the 6,301 people sampled in both 2000 and 2006 cross sectional surveys, 1,044 (17%) were asymptomatically infected with P. falciparum. DNA was extracted from the 1,044 P. falciparum positive bloodspots and PCR amplification of dhfr and dhps performed, giving a combined rate of PCR amplification success of 73% for both genes (Table 2). The amplified products were screened for all the variant sequences described in Table 1. Out of the 774 isolates which amplified successfully for dhfr, 84% were single or majority genotype infections and the point mutation haplotypes could easily be determined. Of the 768 samples which amplified successfully for dhps, 77% were single or majority genotype with determinable haplotypes.
Allelic Haplotypes at dhfr and dhps genes
Three point mutations, N51I, C59R and S108N were found in the dhfr gene. Their haplotypic arrangements were CNCSI, CNCNI, CNRNI, CICNI and CIRNI. This pattern of point mutations is common throughout east Africa and has been previously reported in Tanzania16, Malawi19, Kenya20, 21 and Uganda22, 23. One rare combination of mutation; N51I with C59R (CIRSVI) (previously reported in Uganda22) was found in two individuals in this study.
Five dhps mutations (S436A, S436F, S436C, A437G, and mely low frequency and have not been reported before, presumably because of their rarity. This is a reflection of the larger sample size collected in this study and the fact that some alternative typing methodologies do not have the capacity to routinely detect codon 436 SNP's, which normally occurs in a very rare frequency. Most of the typing methodologies routinely detect codons 437 and 540 SNP's.
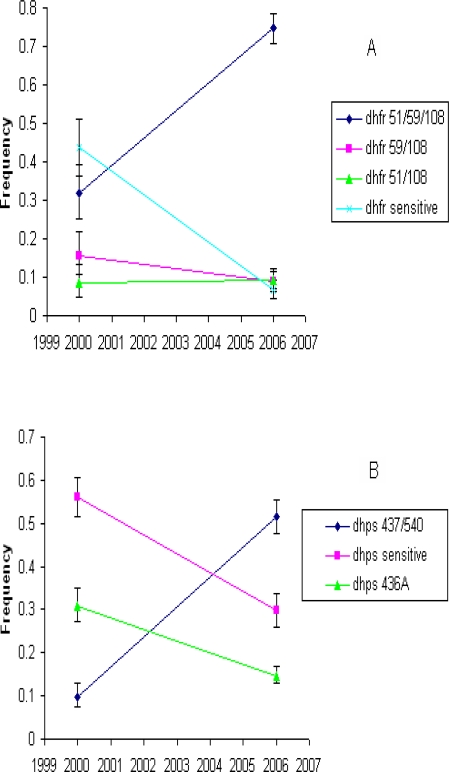
The dhfr and dhps allelic frequency change over time in Morogoro was estimated by comparing isolates sampled at two time points, 2000 and 2006 (Figure 1). The dhfr triple mutant (CIRNI) and the dhps double mutant (SGEAA) haplotypes are the two alleles with greatest significance for SP efficacy. During the 2000 – 2006 period, the triple mutant dhfr allele frequency rose from 0.32 to 0.75 (P≤ 0.0001), dramatically displacing the sensitive dhfr allele frequency from 0.44 to 0.07 (P≤ 0.0001). The escalation of dhfr triple mutant in the population was also associated with significant displacement of dhfr double mutant (CNRN) haplotype frequency from 0.16 to 0.09 (P≤ 0.02). However, the dhfr double mutant (CICN) haplotype, did not change maintaining a constant frequency of 0.09. At the dhps gene, there was an escalation of the double mutant (SGEAA) haplotype by a five fold increase from 0.10 to 0.52 (P≤ 0.0001), displacing both the sensitive dhps and a single mutant (S436A) dhps haplotype from 0.56 to 0.30 (P≤ 0.0001) and from 0.31 to 0.15 (P≤ 0.0001), respectively (Figure 1b).
Figure 1.
Temporal allele frequency trend in Morogoro. A) dhfr alleles and B) dhps alleles
Two Locus Genotypes
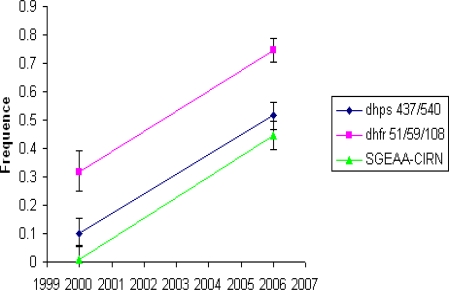
In a further subset of samples for which dhfr and dhps haplotypes could both be conclusively determined it was possible to measure the frequency of two locus genotype combinations of double dhps-triple dhfr (SGEAA-CIRN) mutant. Figure 2 shows change in frequency of this genotype in Morogoro population. Out of 98 dhfr - dhps two locus genotype combinations identified in 2000, only one was SGEAA-CIRN, while in 2006, 169 of the 380 dhfr - dhps two locus genotype combinations were SGEAA-CIRN. This estimate indicates a 45 fold increase of this genotype (1% – 45%) in six years time period. The SGEAA-CIRN genotype is predictive of in vivo SP failure24.
Figure 2.
Temporal trend of the alleles with greatest significance for SP efficacy, the double dhfr and triple dhfr mutant and their combination in Morogoro
Linkage Disequilibrium
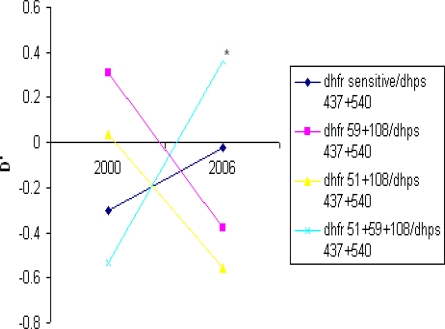
To examine the effects of simultaneous selection by pyrimethamine on dhfr and sulphadoxine on dhps we looked at two-locus genotypes sampled from Morogoro-Mvomero population in the two surveys. Taking the subset of samples for which point mutation haplotypes could be unequivocally resolved at both genes, we compared the observed with expected frequencies generated from contingency tables. Significant association between the dhfr triple mutant allele and the dhps double mutant allele occurred in 2006 but not in 2000 survey. The d' value an index of linkage disequilibrium are shown in Figure 3 which examines linkage between all dhfr alleles and the dhps A437G+K540E double mutant allele. There was a non-significant negative association between dhfr triple mutant allele and the dhps double mutant allele, and non-significant positive associations between dhfr double mutant alleles and the dhps double mutant allele in 2000. The strength of the association between dhfr triple mutant and dhps double mutant alleles increased in 2006 with ≤ 0.36 and chi square analysis showed this was highly significant (pd”0.0001 ). The non-significant positive association between dhfr double mutant alleles and dhps double mutant alleles during 2000 became negative in 2006 (Fig. 3).
Figure 3.
Linkage disequilibrium between the dhps A437G+K540E allele and dhfr alleles The d' values for 2000 (n = 98) and for 2006 (n= 380) are shown. Significant deviation between observed from expected occurred in 2006 indicated by *(p<0.0001)
Discussion
We have demonstrated the SP drug pressure-driven escalation of sulfadoxine and pyrimethamine resistance in Morogoro-Mvomero district in south eastern Tanzania between 2000 – 2006. This escalation was coincident with change of national policy from CQ to SP first line treatment of uncomplicated malaria in August 2001, leading to shift in treatment practice as reported by house-hold survey of antimalarial drug use, which indicated sharp rise of SP use25.
Morogoro and Mvomero are two of the six districts making up the Morogoro region. They both were one district of Morogoro when we conducted the 2000 survey, but later in 2002 divided into two and hence are broadly similar in many aspects. They are well matched in terms of predicted intensity and duration of malaria transmission and risk (MARA), relative access and overall utilization of health services such as IMCI (based on surveys) and fairly usage of insecticide treated nets (ITNs).
SP was approved a national second line treatment of uncomplicated malaria in 1983. Our data indicate it took 17 years of SP second line use for the most resistant triple dhfr-double dhps mutant genotype selection to attain a frequency of 1% in Morogoro-Mvomero population in 2000. Contrastingly, it took only five years for the genotype to rise to 45% under SP first line selection (2001 – 2006) (Pd”0.0001). The frequency of triple mutant dhfr and double mutant dhps resistance alleles rose by 32% and 10%, respectively in 17 years of SP second line use, but the selection strength was more potent during its first line use where the two resistant alleles rose rapidly by 43% and 42% in only five years (Pd”0.0001). The increase of both the double mutant dhps and the triple mutant dhfr displaced alleles with lower fitness by virtue of their resistance properties at both genes. At the dhfr locus, the sensitive allele was displaced from 44% to 7% (Pd”0.0001) in five years while the dhfr double mutant (C59R+S108N) was displaced from 16 % to 9% (Pe”0.05). Surprisingly the dhfr double mutant (N51I+ S108N) remained unchanged throughout maintaining a constant frequency of 10%. At the dhps locus, the rise of double mutant allele (A437G+K540E) displaced the sensitive allele from 56% to 30% (Pd”0.0001) and the single mutant allele (S436A) from 30% to 15% (Pd”0.0001). The contrasting response of both dhfr and dhps alleles to the increase of SP selection pressure following its widespread use as first line treatment of malaria, likely reflect their different contribution to the SP resistance. Under the population genetics perspective, for the successful spread of a resistance allele, it must be transmitted at a faster rate than the sensitive form. Since the transmission of both double mutant dhfr and single mutant dhps S436A was by far lower than the dhfr triple and dhps double mutant alleles, it is likely that they play a peripheral or transient role in the pyrimethamine and sulfadoxine resistance.
LD between the dhfr triple and dhps double mutants is an indirect measure of effective selection pressure. The combination of the two alleles confers greater fitness to the parasite than each allele separately, thus enhancing the chance of better survival of the parasite in the face of SP drug pressure. The two locus combination of the dhfr triple mutant and the dhps double mutant has been shown to have statistical association with treatment failure21, 24, 26. The LD measured in 2000 indicates it was not statistically significant and its interpretation suggests the selection applied when SP was a second line was insufficient to keep the two unlinked alleles in association. By contrast, a strong association between the two alleles emerged in the 2006, confirming potent selection strength applied by SP first line. We predict that, following withdraw of SP as national first line atimalarial drug, the drug selection pressure will most likely decrease substantially as SP use remain limited to specific programmes only. This decrease in drug selection pressure will disrupt the association between the triple mutant dhfr and double mutant dhps alleles leading to loss of LD.
Acknowledgement
We are grateful to the people of Mvomero and Morogoro districts who participated in the study. We also thank the IMPACT-Tz team members who did the surveys in 2000 and 2006. This study was conducted under the auspices of Interdisciplinary Monitoring Project for Antimalarial Combination Therapy in Tanzania (IMPACT-Tz). IMPACT-Tz is primarily supported financially by the United States Agency for International Development (USAID)
Conclusion
This report has clearly shown that the population rate of change of resistant dhfr and dhps alleles is contingent to the SP usage in the population. Greater rate of change was observed when SP was a national first line than when was the second line antimalarial treatment policy. The 2006 dhfr and dhps allele frequencies reported here will be useful in the monitoring of rate of reversal of resistant forms of these alleles following withdrawal of SP use as a first linetreatment in Tanzania in the late 2006.
References
- 1.Le Sueur D, Binka F, Lengeler C, de Savigny D, Snow RW, Teuscher T, Toure Y. Africa Health. Cambridge, UK: FSG Medi Media Ltd; 1998. An atlas of malaria in Africa; pp. 23–24. [PubMed] [Google Scholar]
- 2.Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, et al. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 3.Roper C, Pearce R, Nair S, Sharp B' Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science 2004 (Wash DC) 305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 4.Pearce R, Malisa A, Kachur SP, Barnes K, Sharp B, Roper C. Reduced Variation Around Drug Resistant dhfr Alleles in African Plasmodium falciparum. Molecular Biology and Evolution. 2005;22:1834–1844. doi: 10.1093/molbev/msi177. [DOI] [PubMed] [Google Scholar]
- 5.Ndiaye D, Daily JP, Sarr O, Ndir O, Gaye O, Mboup S, et al. Defining the origin of Plasmodium falciparum resistant dhr isolates in Senegal. Acta Tropica. 2006;99:106–111. doi: 10.1016/j.actatropica.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCollum AM, Poe AC, Hamel M, Huber C, Zhou Z, Shi YP, et al. Antifolate Resistance in Plasmodium falciparum: Multiple Origins and Identification of Novel dhfr Alleles. Journal of Infectious Diseases. 2006;194:189–197. doi: 10.1086/504687. [DOI] [PubMed] [Google Scholar]
- 7.Maiga O, Djimde AA, Hubert V, Renard E, Abouy A, Kironde F, et al. A shared Asian Origin of the Triple-Mutant dhfr Allele in Plasmodium falciparum from sites across Africa. Journal of Infectious Diseases. 2007;196:165–172. doi: 10.1086/518512. [DOI] [PubMed] [Google Scholar]
- 8.Lynch C, Pearce R, Pota H, Cox J, Abeku TA, Rwakimari J, et al. Emergence of a dhfr Mutation Conferring High-Level Drug Resistance in Plasmodiumfalciparum Populations from Southwest Uganda. Journal of Infectious Diseases. 2008;197:1598–1604. doi: 10.1086/587845. [DOI] [PubMed] [Google Scholar]
- 9.Certain LK, Briceno M, Kiara SM, Nzila AM, Watkins WM, Sibley CH. Characteristics of Plasmodium falciparum dhfr haplotypes that confer pyrimethamine resistance, Kilifi, Kenya, 1987–2006. Journal of Infectious Diseases. 2008;197:1743–1751. doi: 10.1086/588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugittu K, Ndejembi M, Malisa A, Lemnge M, Premji Z, Mwita A, et al. Therapeutic Efficacy of Sulfadoxine-pyrimethamine and Prevalence of Resistance Markers in Tanzania Prior to Revision of Malaria Treatment Policy: Plasmodium falciparum Dihydrofolate Reductase and Dihydropteroate Synthase Mutations in Monitoring in vivo Resistance. Am J Trop Med Hyg. 2004;71(6):696–702. [PubMed] [Google Scholar]
- 11.Mugittu K, Abdulla S, Falk N, Masanja H, Felger I, Mshinda H, et al. Efficacy of Sulfadoxine-Pyrimethamine in Tanzania after two years as first-line drug for uncomplicated malaria: assessment protocol and implication for treatment policy. Malaria Journal. 2005;4:55. doi: 10.1186/1475-2875-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, Tanner M, et al. Intermittent treatment for malaria and anaemiacontrol at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet. 2001;357:1471–1477. doi: 10.1016/S0140-6736(00)04643-2. [DOI] [PubMed] [Google Scholar]
- 13.Menendez C, Schellenberg D, Macete E, Aide P, Kahigwa E, Sanz S, et al. Varying efficacy of intermittent preventive treatment for malaria in infants in two similar trials: public health implications. Malar J. 2007;6:132. doi: 10.1186/1475-2875-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosling RD, Ghani AC, Deen JL, Seidlein L, Greenwood BM, Chandramohan D. Can changes in malaria transmission intensity explain prolonged protection and contribute to high protective efficacy of intermittent preventive treatment for malaria in infants? Malaria J. 2008;7:54. doi: 10.1186/1475-2875-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, et al. Return of Chloroquine Antimalarial Efficacy in Malawi. N Engl J Med. 2006;355(19):1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 16.Pearce R, Drakeley C, Chandramohan D, Mosha F, Roper C. Molecular Determination of Point Mutation Haplotypes in the Dihydrofolate Reductase and Dihydropteroate Synthase of Plasmodium falciparum in Three Districts of Northern Tanzania. Antimicrobial Agents and Chemotherapy. 2003;47:1347–1354. doi: 10.1128/AAC.47.4.1347-1354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conway DJ, Roper C, Oduola AM, Arnot DE, Kremsner PG, Grobusch MP, et al. High recombination rate in natural populations of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1999;96:4506–4511. doi: 10.1073/pnas.96.8.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider S, Roessli D, Excoffier L. Arlequin ver. 2.000: software for population genetics data analysis version 2.000 edition. Geneva, Switzerland: Genetics and Biometry Laboratory, University of Geneva; 2000. [Google Scholar]
- 19.Bwijo B, Kaneko A, Takechi M, Zungu IL, Moriyama Y, Lum JK, et al. High prevalence of quintuple mutant dhfr/dhps genes in Plasmodium infections seven years after introduction of sufadoxine and pyrimethamine as first line treatment in Malawi. Acta Tropica. 2003;85:363–373. doi: 10.1016/s0001-706x(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 20.Nzila AM, Mberu EK, Sulo J, Dayo H, Winstanley PA, Sibley CH, Watkins WM. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: Genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrobial Agents and Chemotherapy. 2000;44(4):991–996. doi: 10.1128/aac.44.4.991-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omar SA, Adagu IS, Warhurst DC. Can pretreatment screening for dhps and dhfr point mutations in Plasmodium falciparum infections be used to predict sulfadoxine-pyrimethamine treatment failure? Trans Roy Soc Trop Med Hyg. 2001;95:315–319. doi: 10.1016/s0035-9203(01)90250-0. [DOI] [PubMed] [Google Scholar]
- 22.Kyabayinze D, Cattamanchi A, Kamya MR, Rosenthal PJ, Dorsey G. Validation of a simplified method for using molecular markers to predict sulfadoxine-pyrimethamine treatment failure in African children with falciparum malaria. Am J Trop Med Hyg. 2003;69(3):247–252. [PubMed] [Google Scholar]
- 23.Sendagire H, Kyabayinze D, Swedberg G, Kironde F. Plasmodium falciparum: higher incidence of molecular resistance markers for sulphadoxine than for pyrimethamine in Kasangati, Uganda. Trop Med Int Health. 2005;10(6):537–543. doi: 10.1111/j.1365-3156.2005.01414.x. [DOI] [PubMed] [Google Scholar]
- 24.Kublin JK, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–388. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- 25.Goodman C, Kachur SP, Abdulla S, Mwageni E, Nyoni J, Schellenberg JA, et al. Retail supply of malaria-related drugs in rural Tanzania: risks and opportunities. Trop Med IntHealth. 2004;9(6):655–663. doi: 10.1111/j.1365-3156.2004.01245.x. [DOI] [PubMed] [Google Scholar]
- 26.Steadke SG, Sendagire H, Lamola S, Kamya MR, Dorsey G, Rosenthal PJ. Relationship between age, molecular markers, and response to sulphadoxine-pyrimethamine treatment in Kampala, Uganda. Trop Med Int Health. 2004;9(5):624–629. doi: 10.1111/j.1365-3156.2004.01239.x. [DOI] [PubMed] [Google Scholar]