Abstract
Striated muscle cells display an extremely regular assembly of their actin cytoskeleton that contributes to the contractile elements, the myofibrils. How this assembly is initiated and how these structures are maintained is still unclear. We have recently shown that striated muscle expresses a specific isoform of the formin protein family member FHOD3, which is characterised by the presence of a CK2 phosphorylation site at the C-terminal end of the formin homology domain 2 (FH2). Phosphorylated muscle FHOD3 displays a different subcellular localisation, namely to the myofibrils, and also has increased stability compared to un-phosphorylated or non muscle FHOD3. In addition, we could show that muscle FHOD3 is involved in myofibril maintenance in cultured cardiomyocytes and that its presence dramatically enhances the reconstitution of cardiac actin filaments after depolymerisation. Since FHOD3 expression levels and in particular that of the muscle isoform are also decreased in different types of cardiomyopathy, we postulate a crucial role for this protein in the maintenance of a fully functional cardiac cytoarchitecture.
Key words: heart, development, actin filament, formin, sarcomere
Striated (cardiac and skeletal) muscle cells are characterised by their extremely regular cytoarchitecture. Actin and myosin are assembled into contractile elements that are arranged into para-crystalline myofibrils by a multitude of associated proteins (Fig. 1A).1,2 Currently it is still unclear, how exactly this precise assembly is regulated and also how protein turnover, either during normal maintenance or during adaptation to different working conditions e.g., by the swapping of isoforms, is achieved.3 Recent work has taken an inspiration from decades of research on the regulation of the actin cytoskeleton in migrating fibroblasts, where two major ways of controlling F-actin assembly were described: the ARP2/3 complex and its activator N-WASP leads to the formation of branched actin filaments, while different members of the formin protein family stimulate the assembly of unbranched actin filaments.4,5
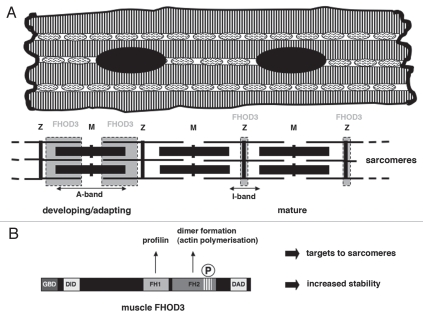
Figure 1.
(A) Schematic drawing of a cardiomyocyte, showing the myofibrils as stripes with the mitochondria in-between, nuclei are black and the special type of cell-cell contact, the intercalated disc, is shown as a thick vertical wavy line. Below is a schematic representation of a myofibril, composed of individual sarcomeres. A sarcomere is defined as the region between two Z-discs (Z), in which the thin (actin) filaments are anchored (depicted as thin horizontal lines). Muscle contraction is brought about by interaction of thin filaments with the thick (myosin) filaments (depicted as bold horizontal lines), which are anchored in the middle of the sarcomere in a structure called the M-band (M). In developing muscle cells or in cardiomyocytes that adapt to culture conditions FHOD3 is located in a broad double band (shown in grey) in the sarcomere, while in freshly isolated mature cardiomyocytes or in heart tissue sections it is restricted to the Z-disc region (thin grey areas). (B) Schematic representation of the muscle FHOD3 isoform. The eight amino acid long insertion that originates from the muscle specific exon is depicted as stripes at the end of the formin homology (FH) 2 domain. The P refers to its phosphorylation. Interaction of the FH1 domain with profilin and the potential of dimer formation of FH2 domains, which is essential for actin polymerisation, are indicated above the molecule. GBD GTPase binding domain, DID diaphanous inhibitory domain, DAD diaphanous autoinhibitory domain.
It now begins to emerge that the assembly or maintenance of actin filaments in muscle cells may actually involve well-known players from cell migration.6–8 For example, in a paper that was recently published, we were able to show that a striated muscle specific splice variant of the formin FHOD3 plays a crucial role in the maintenance of actin filaments in cardiomyocytes in culture.6 The muscle isoform of FHOD3 is characterised by the inclusion of an additional exon that encodes for eight highly conserved amino acids at the C-terminal end of the formin homology 2 (FH2) domain and thereby introduces a CK2 phosphorylation site (Fig. 1B). This phosphorylation by CK2, which is a constitutively active and ubiquitously expressed kinase,9 has dramatic consequences on the subcellular localisation of muscle FHOD3, since it gets targeted to the myofibrils, and also on the protein stability, which is increased. Both aspects were also confirmed by using phosphomimicking constructs or constructs that could not be phosphorylated.6 An effect of phosphorylation by CK2 on protein stability and protein-protein interaction and thus subcellular localisation was previously already observed for e.g., the phosphatase PTEN or for beta-catenin,9,10 but this is to our knowledge the first time that a similar effect is seen for a cytoskeletal protein in muscle cells. Apart from its obvious consequences for protein stability and subcellular targeting, it is unclear at the moment, what kind of effect the phosphorylation would have on the function of FHOD3. Structural studies have established that two FH2 domains have to dimerise to a doughnut-like structure to enable actin polymerisation (for review including a very instructive movie see 11) and the CK2 site sits at the C-terminal end of the FH2 domain of FHOD3. CK2 phosphorylation has been suggested as an alpha-helix breaker12 and could have a similar effect in the case of muscle FHOD3. However, even if the alpha-helix is broken, judged by actin polymerization experiments that we carried out in HeLa cells, there is no distinguishable difference on the efficiency of muscle FHOD3 at least in that particular system.6 Due to our inability to express soluble recombinant FH1-FH2 constructs, we were not able to explore any potential differences in in vitro actin polymerisation assays. Therefore the detailed kinetic characterization of muscle versus non muscle FHOD3 in F-actin assembly as well as any effect on the 3D structure of the FH2 dimer doughnut remain to be investigated.
It is extremely intriguing that endogenous FHOD3 seems to be located in different parts of the sarcomere, depending, whether mature cardiomyocytes in situ or neonatal cardiomyocytes in culture are analyzed.6 In both mouse and human heart as well as in freshly isolated adult rat cardiomyocytes FHOD3 is found at the Z-disc, while in neonatal rat cardiomyocytes a broader localization as a doublet is observed, which is also seen for epitope-tagged full length FHOD3 (grey signal in Fig. 1A). A similar broader localisation is detected in embryonic mouse hearts (Iskratsch and Ehler, manuscript in preparation). We speculate that the Z-disc localization may be the default targeting in the “resting state” with only comparatively little thin filament turnover. The broader localization may reflect FHOD3 being busily involved in actin assembly. A more dynamic state of thin filaments in culture may explain the extremely rapid recovery of the actin cytoskeleton in neonatal rat cardiomyocytes (NRC) after disassembly, as apparent in our latrunculin B experiments. Already after 30 minutes reasonably restored actin filaments were seen, although as apparent from our RNAi knockdown experiments, only if FHOD3 was present in the system. Side to side comparison also revealed that at least under these conditions muscle FHOD3 is much more efficient than another formin, mDia-1, in the rescue.6 NRC have to rearrange their myofibrils in order to adapt from the 3D conditions in situ to the more or less 2D conditions in the culture dish and the presence of an extremely rapidly exchanging F-actin population in these cells was also reported by others.13
While the beneficial effect of FHOD3 on myofibril maintenance is obvious from our results and also from previous studies by Taniguchi and colleagues,14 it is still unclear whether FHOD3 or any other formin plays a crucial role during myofibrillogenesis in the embryo. We have observed FHOD3 expression from early on in the mesoderm of chick embryos and in mouse hearts at E9.5, when beating has just started (Iskratsch and Ehler, manuscript in preparation), but currently it is not known whether the presence of FHOD3 is essential for initial myofibril assembly. It may well be that several formins are involved, since recent data on Daam-1 (another member of the formin protein family) deficient mice showed impaired cardiac morphogenesis and also reduced myofibril assembly.15 However, from the preferential subcellular targeting of Daam-1 to the plasma membrane and its effect on the localization of alpha-catenin and N-cadherin in the adherens junctions of the embryonic cardiomyocytes, it appears more likely that Daam-1 plays a crucial role in the organization of the actin membrane cytoskeleton and the myofibril anchorage at these sites than directly in sarcomerogenesis.
While our work has focused on the role in myofibril maintenance of a formin that is highly expressed in the heart, others could show that N-WASP, which is another well known player in the regulation of the actin cytoskeleton via the Arp2/3 complex during cell migration, is also beneficial for myofibril growth, at least in skeletal muscle.8 N-WASP and formins share a similar mechanism of control of activity: in the inactive state an intramolecular interaction between the N-terminus and the C-terminus of the protein prevents access to the active sites in the middle of the molecule. This interaction can be relieved for example by the binding of small GTPases (Cdc42 for N-WASP, Rho/Rac-1 for formins).16 Interestingly, in skeletal muscle N-WASP gets activated by a different way, namely the interaction of its proline-rich domain with the SH3 domain of a striated muscle specific protein, nebulin and its function in this context appears to be independent from the Arp2/3 complex. Also N-WASP appears to be localized to different parts of the sarcomere in skeletal muscle cells, depending on the extent of myofibrillogenesis: at resting state it is found at the Z-disc, while following growth factor stimulation and therefore increased myofibril assembly its localization extends along the thin filament towards the M-band region.8 The Z-disc region of skeletal muscle is also, where gamma-cytoskeletal actin can be found in addition to muscle actin isoforms, apparently playing a role in long term muscle maintenance.17 Both the localization behavior of N-WASP and its intramolecular inhibition are reminiscent of FHOD3 and it seems to be likely that also other stalwarts of actin regulation during cell migration in fibroblasts may be involved in the process of myofibril assembly and maintenance. As with skinning cats, there would seem to be more than one way to maintain thin filaments in a muscle cell.
Acknowledgements
Work in the laboratory of Elisabeth Ehler was supported by a Medical Research Council Career Establishment Grant. Thomas Iskratsch was the recipient of a King's College London Strategic Investment Ph.D., Fellowship that was awarded to the Cardiovascular Division and a British Heart Foundation Research Excellence Centre Pump Priming Fellowship.
Abbreviations
- 3D
three-dimensional
- CK
formerly known as casein kinase
- FH
formin homology
- NRC
neonatal rat cardiomyocytes
References
- 1.Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- 2.Ehler E, Gautel M. The sarcomere and sarcomerogenesis. Adv Exp Med Biol. 2008;642:1–14. doi: 10.1007/978-0-387-84847-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Sparrow JC, Schöck F. The initial steps of myofibril assembly: integrins pave the way. Nat Rev Mol Cell Biol. 2009;10:293–298. doi: 10.1038/nrm2634. [DOI] [PubMed] [Google Scholar]
- 4.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 5.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iskratsch T, Lange S, Dwyer J, Kho AL, dos Remedios C, Ehler E. Formin follows function: a muscle-specific isoform of FHOD3 is regulated by CK2 phosphorylation and promotes myofibril maintenance. J Cell Biol. 2010;191:1159–1172. doi: 10.1083/jcb.201005060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautel M, Ehler E. Cell biology. Gett'N-WASP stripes. Science. 2010;330:1491–1492. doi: 10.1126/science.1199920. [DOI] [PubMed] [Google Scholar]
- 8.Takano K, Watanabe-Takano H, Suetsugu S, Kurita S, Tsujita K, Kimura S, et al. Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science. 2010;330:1536–1540. doi: 10.1126/science.1197767. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez I, Sonenshein GE, Seldin DC. Protein kinase CK2 in health and disease: CK2 and its role in Wnt and NFkappaB signaling: linking development and cancer. Cell Mol Life Sci. 2009;66:1850–1857. doi: 10.1007/s00018-009-9153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 11.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 12.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? Faseb J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 13.Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J Cell Sci. 2009;122:2119–2126. doi: 10.1242/jcs.046805. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi K, Takeya R, Suetsugu S, Kan OM, Narusawa M, Shiose A, et al. The mammalian formin Fhod3 regulates actin assembly and sarcomere organization in striated muscles. J Biol Chem. 2009;284:29873–29881. doi: 10.1074/jbc.M109.059303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138:303–315. doi: 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells. 2010;29:311–325. doi: 10.1007/s10059-010-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kee AJ, Gunning PW, Hardeman EC. Diverse roles of the actin cytoskeleton in striated muscle. J Muscle Res Cell Motil. 2009;30:187–197. doi: 10.1007/s10974-009-9193-x. [DOI] [PubMed] [Google Scholar]