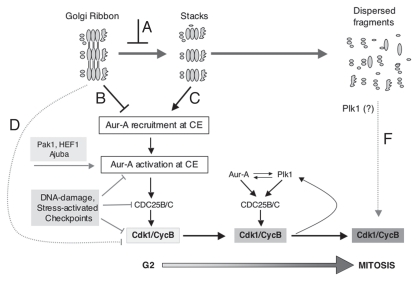
Figure 1.
Unlinking of the Golgi ribbon during G2 inhibits the recruitment and activation of Aur-A at the centrosome trough a novel mechanism. The Golgi complex is organised as a continuous membranous system composed of stacks interconnected by tubules, a structure known as the “Golgi ribbon.” During the G2 phase of the cell cycle, the Golgi ribbon is cleaved into its constituent “stacks.” A block in Golgi fragmentation (A) impairs Aur-A recruitment at the centrosome (CE). This effects on Aur-A can be explained by two models: the triggering of a signalling pathway that causes the release of Aur-A from the centrosomes (B); or the lack of a positive signal that supports the recruitment of Aur-A to the centrosomes (C). The inhibition of Aur-A recruitment and activation at the centrosome in turn impairs the first activation of Cdk1-CycB at the centrosome during early G2 (light grey box). The Golgi-dependent G2 block is not mediated by known Aur-A activators (i.e., Pak1, HEF1, Ajuba), and nor it is indirectly mediated by Plk1 and Cdk1-CycB complex. In addition, the Golgi-dependent G2 block is not regulated by known G2 checkpoint mediators, which act downstream the Golgi checkpoint. At the onset of mitosis the kinases Aur-A, Plk1 and cycB-Cdk1 become functionally connected by a positive feedback loop, leading to irreversible activation of Cdk1-CycB (dark grey box) and progression into mitosis. In the case of a prolonged inhibition of fragmentation of the Golgi ribbon, additional synergistic mechanisms can help to prolong the cell cycle block. For example, a block of Golgi fragmentation could stimulate a transcription-based response that leads to the expression of inhibitors of Cdk1-CycB (D), or it would impede the activation of a Golgi-based Plk1/Cdk1/CycB2 pathway (E).