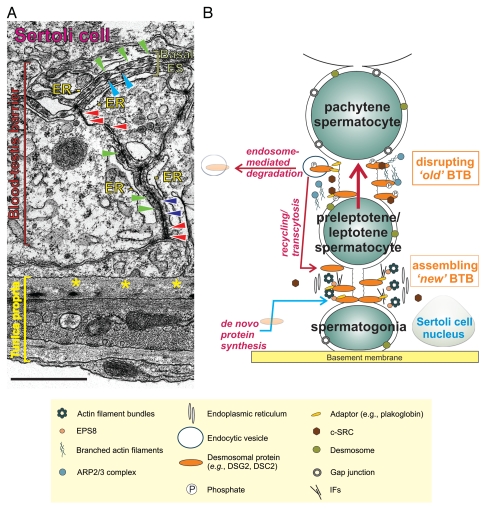
Figure 1.
Morphological features of the desmosome and its intimate relationship with TJs, basal ESs and gap junctions that together constitute the BTB in the mammalian testis. (A) This is an electron micrograph of a cross-section of the seminiferous tubule from an adult rat testis. It shows the seminiferous epithelium, which is composed of Sertoli and developing germ cells resting on the basement membrane (a modified type of extracellular matrix, see yellow asterisks) of the tunica propria. Desmosomes are seen between two Sertoli cells (see red arrowheads); they are typified by the presence of electron dense material. Basal ESs, on the other hand, are typified by the presence of actin filament bundles (see green arrowheads) sandwiched in between cisternae of endoplasmic reticulum (ER) and the Sertoli cell plasma membrane. Basal ESs co-exist with either TJs (“kisses” between apposing Sertoli cell plasma membranes, see blue arrowheads) or gap junctions (see purple arrowheads). Thus, desmosomes are critical structural components that constitute BTB function. Bar = 1 µm. (B) This is a schematic drawing of the seminiferous epithelium illustrating the relative location of the BTB. Desmosomal proteins (e.g., DSG2, DSC2, IFs) at the BTB may be phosphorylated by c-SRC, thereby inducing their internalization via endocytic vesicles. Internalized proteins may be degraded, thereby destabilizing the “old” BTB to facilitate the transit of preleptotene spermatocytes. Additionally, desmosomes also serve as a platform for signal transduction events. For instance, c-SRC -mediated protein phosphorylation may further destabilize the BTB. Internalized proteins may also be recycled and trafficked to the “new” BTB to establish new TJ-fibrils. Coupled with de novo synthesis of TJ proteins, the integrity of the immunological barrier can be maintained during the transit of preleptotene spermatocytes.