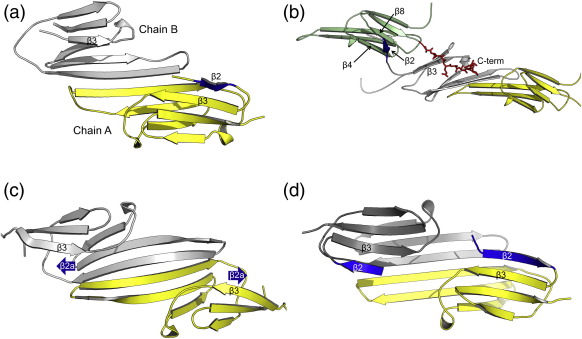
Fig. 3.
Variability and strand swapping at the β3:β2 position. (a) In human αB (2WJ7) the amino acids adjacent to β3 form the β2 strand in one chain of the dimer. (b) In rat Hsp20 (2WJ5) the β2 strand is not part of a β-sheet and (together with the construct N-terminus) is extended in both chains, allowing a hydrophobic side chain to bind in the β4/β8 pockets of an adjacent dimer. The C-terminal extension of a symmetry-related chain now binds to the β3 strand (shown as red sticks). (c) In human αB (2KLR) the intrachain β2 strands are not present; however, interactions from upstream N-terminal residues from another dimer are present. (d) The R120G structure showing intrachain β2 strands for each chain.