Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is an oncogenic retrovirus that is etiologically associated with adult T-cell leukemia. The HTLV-1 bZIP factor (HBZ), which is encoded by the minus strand of the provirus, is involved in both regulation of viral gene transcription and T-cell proliferation. We showed in this report that HBZ interacted with Smad2/3, and enhanced transforming growth factor-β (TGF-β)/Smad transcriptional responses in a p300-dependent manner. The N-terminal LXXLL motif of HBZ was responsible for HBZ-mediated TGF-β signaling activation. In a serial immunoprecipitation assay, HBZ, Smad3, and p300 formed a ternary complex, and the association between Smad3 and p300 was markedly enhanced in the presence of HBZ. In addition, HBZ could overcome the repression of the TGF-β response by Tax. Finally, HBZ expression resulted in enhanced transcription of Pdgfb, Sox4, Ctgf, Foxp3, Runx1, and Tsc22d1 genes and suppression of the Id2 gene; such effects were similar to those by TGF-β. In particular, HBZ induced Foxp3 expression in naive T cells through Smad3-dependent TGF-β signaling. Our results suggest that HBZ, by enhancing TGF-β signaling and Foxp3 expression, enables HTLV-1 to convert infected T cells into regulatory T cells, which is thought to be a critical strategy for virus persistence.
Introduction
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia (ATL) and HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP).1,2 The unique sequence of HTLV-1 between the env region and 3′ long terminal repeat, denoted the pX region, encodes regulatory (tax and rex) and accessory (p12, p13, and p30) genes in the plus strand.3 The pleiotropic actions of Tax are thought to play a central role in the early stage of leukemogenesis.4 However, approximately 60% of fresh ATL cells lack Tax expression because of genetic and epigenetic changes in the HTLV-1 provirus, suggesting that Tax may not be essential for the maintenance of ATL cells in vivo.5 The HTLV-1 bZIP factor (HBZ), which is encoded by the complementary strand of the HTLV-1 genome, is expressed in all ATL cases and supports the proliferation of ATL cells.6,7 In addition, HBZ was found to inhibit Tax-mediated activation of viral transcription from the 5′-long terminal repeat by heterodimerizing with c-Jun, CREB2, and to selectively suppress the classic pathway of nuclear factor-κB by 2 distinct mechanisms.8–11 Thus, HBZ has multifunctional roles in cellular signaling and proliferation. Recently, we reported that nonsense mutations in all HTLV-1 genes except HBZ were generated by APOBEC3G before integration of the provirus in ATL cases and HTLV-1 infected persons, indicating that the HBZ gene is essential for leukemogenesis.12
Transforming growth factor-β (TGF-β) controls a variety of biologic processes, including cell growth, differentiation, apoptosis, development, and immune homeostasis.13 The Smad proteins, which are mediators of TGF-β signaling, transduce the TGF-β signal at the cell surface into gene regulation in the nucleus. Receptor-regulated Smad, R-Smads (Smad1, 2, 3, 5, and 8), are phosphorylated by the activated TGF-β receptor, form complexes with Comediator Smad, Co-Smad (Smad 4), and together accumulate in the nucleus to regulate transcription of target genes. Smads can regulate gene expression positively by recruiting coactivators, such as CBP/p300,14 or negatively by direct recruitment of histone deacetylases or corepressors, such as c-Ski and SnoN.15 In ATL cells, constitutively activated AP-1 leads to the production of TGF-β1,16 which can be readily detected in the serum of infected persons.17 Subsequent studies reported that HTLV-1–infected T cells were resistant to TGF-β–induced growth inhibition and that resistance was related to Tax expression. Three distinct mechanisms by which Tax suppressed TGF-β–mediated signaling were reported: (1) inhibition of Smad3-Smad4 complex formation and DNA binding; (2) prevention of the recruitment of CBP/p300 to the Smad transcription complex on TGF-β response elements; and (3) inhibition of Smad3 DNA binding through activation of the JNK/c-Jun pathway.18–20
TGF-β signaling is critical for the development of CD4+CD25+Foxp3+ regulatory T cells (Tregs).21 ATL cells possess a CD4+CD25+ phenotype, similar to that of Tregs. The forkhead box P3 (FoxP3) is critical for the function of Tregs.22 Expression of FoxP3 was detected in two-thirds of ATL cases,23 indicating that ATL cells are derived from Tregs, and a recent study reported a higher proportion of FoxP3+ Tregs among the HTLV-1–infected cells than among the HTLV-1–negative CD4+ cells.24 Although Tax has been reported to suppress FoxP3 expression in T cells,25 we recently found that HBZ expression-induced Foxp3 expression and increased the number of Tregs.26 Therefore, we set out to determine how HBZ and TGF-β cooperate to induce FoxP3 in HTLV-1–infected T cells.
In the present study, we found that HBZ enhanced TGF-β signaling by interacting with Smad3 and p300, resulting in enhanced Foxp3 expression. This might account for why HTLV-1 infection increases Tregs in vivo.
Methods
Cell culture and mice
HTLV-1 immortalized cell lines (MT-2, MT-4), ATL cell lines (MT-1, ATL-2, ATL-T, ATL-43T, ATL-55T, ED, and TL-Om1), T-cell lines not infected with HTLV-1 (Jurkat, Hut78, CEM, and Kit 225), and mouse T-cell line (CTLL-2) were maintained as described previously.27 HepG2, 293T, and COS7 cells were grown in Dulbecco modified Eagle medium supplemented with 10% FBS and antibiotics. Plat-E cells were cultured in Dulbecco modified Eagle medium supplemented with 10% FBS containing 10 μg/mL blasticidin and 1 μg/mL puromycin. EL4 cells were cultured with RPMI 1640 containing 10% FCS, antibiotics, and 50μM 2-mercaptoethanol. C57BL/6J mice were purchased from CLEA Japan. Peripheral blood mononuclear cells were isolated from healthy volunteers under an institutional review board-approved protocol. All animals used in this study were maintained and handled according to protocols approved by Kyoto University.
Plasmids
The 3TP-Lux construct contains the phorbol myristate acetate-response elements along with the Smad3/4 binding sites of the PAI-1 promoter. A total of 9 × CAGA-Luc contains 9 tandem Smad3/4 binding sites. pTARE-Luc was purchased from Stratagene. phRL-TK was purchased from Promega. Expression plasmids for Tax, spliced HBZ (sHBZ), unspliced HBZ (usHBZ), c-Ski, Smads, and their deletion mutants were prepared as previously described.11,28 Expression vectors for sHBZ-ΔbZIP deletion mutants were generated by polymerase chain reaction (PCR). The coding region of c-Fos was amplified by reverse-transcribed (RT)-PCR from total RNA derived from primary peripheral blood mononuclear cells and cloned into the vector pCDNA3. Expression plasmids for E1A and its mutants were a gift from Dr Akiyoshi Fukamizu of University of Tsukuba, and pEF-HA-p300 was from Dr Takashi Fujita of Kyoto University. The STAT5-responsive Jγ1 promoter reporter plasmid (pGL4-Jγ1), pCAGGS-WT-STAT5a (wild-type), and pCAGGS-CA-STAT5a (constitutively active) were provided by Dr Koichi Ikuta of Kyoto University.
Luciferase assay
HepG2 cells were plated on 12-well plates at 2.5 × 105 cells per well. After 24 hours, cells were transfected with the indicated plasmid. At 48 hours after transfection, a luciferase reporter assay was performed as previously described.11 For CTLL-2 cells, vectors were transfected with a Gene Pulser II electroporation system (Bio-Rad). The transfected cells were treated with or without 10 ng/mL TGF-β (RD Systems) for 24 hours before being harvested for the reporter assay. For the Foxp3 reporter assay, the Foxp3 gene promoter and enhancer were cloned into the pGL4.1 basic vector as previously reported.29 Mutations of the Smad binding site in the enhancer fragment were generated by PCR. Foxp3 reporter assays were performed in EL4 cells as described.29 Each experiment was performed in triplicate, and the data represent the mean plus or minus SD of 3 independent experiments, each normalized to Renilla activity.
Immunoprecipitation and immunoblotting
HepG2 and COS7 cells were transfected with the indicated plasmids by TransIT-LT1 (Mirus). Tagged proteins were isolated from transfected COS7 cells by immunoprecipitation with anti–c-Myc (clone 9E10, Sigma-Aldrich), anti-HA (12CA5, Roche Diagnostics) or anti-FLAG M2 (Sigma-Aldrich) antibodies, and analyzed by Western blot as described previously.11
For sequential immunoprecipitation, transfected COS7 cells were lysed in radioimmunoprecipitation assay buffer and immunoprecipitated with anti-FLAG M2 agarose affinity gel. The precipitates were eluted with FLAG peptide and the eluate diluted with radioimmunoprecipitation assay buffer, immunoprecipitated with anti-HA antibody, and subjected to anti-His (MBL) immunoblotting. Membranes were developed by enhanced chemiluminescence (GE Healthcare Life Sciences). Other antibodies used were as follows: anti–mouse immunoglobulin G (IgG), and anti–rabbit IgG were from GE Healthcare Life Sciences; anti-Smad3 was from ZYMED Laboratories (Zymed); and anti-HBZ was used as previously described.30
Immunofluorescence analysis
COS7 cells were transfected with expression vectors using TransIT-LT1 and treated with TGF-β (5 ng/mL) for 2 hours. At 48 hours after transfection, sHBZ protein was detected using anti–c-MYC Cy3 (clone 9E10, Sigma-Aldrich). Smad3 was detected using anti–FLAG-biotin (Sigma-Aldrich) and secondary streptavidin-Alexa-488 antibody (Invitrogen). Fluorescence was observed with a 63×/1.4-0.60 HCX PL APO objective on a DMIRE2-TCS AOBS confocal microscope system (Leica). Images were acquired and analyzed using LCS 2.61 (Leica) and processed using Photoshop CS2 (Adobe Systems).
ChIP assay
HepG2 cells were transfected with the indicated expression vectors together with 3TP-Lux reporter plasmid. At 24 hours after transfection, cells were treated with TGF-β (5 ng/mL). At 48 hours after transfection, ChIP assay was done according to the protocol recommended by Upstate Biotechnology. Precipitated DNA was amplified by PCR using primers specific for 3TP-Lux vector. Sequences for the primer set were 5′-CCCCCTGAACCTGAAACATA-3′ and 5′-CCTGAGGGCTCTCTTGTGTC-3′. For the endogenous FoxP3 enhancer, chromatin samples prepared from MT-2 cells treated with 5 ng/mL of TGF-β for 2 hours were subjected to ChIP analysis using the following antibodies: anti-HBZ,31 anti-Smad3 (BD Biosciences), anti-p300 (Santa Cruz Biotechnology), and normal mouse or rabbit immunoglobulin G (Santa Cruz Biotechnology). Primers used were 5′-CCTATGTTGGCTTCTAGTCTCTTTTATGG-3′ and 5′-TATGGAGA-GGTTAAGTGCCTGGCTA-3′.
Synthesis of cDNA and semiquantitative RT-PCR
Total RNA was isolated using Trizol Reagent (Invitrogen) according to the manufacturer's instructions. We reverse-transcribed 1 μg of total RNA into single-stranded cDNA with SuperScript II reverse transcriptase (Invitrogen). Using forward (F) and reverse (R) primers specific to the target genes, the cDNA was amplified by increasing PCR cycles. The specific primers used can be found in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Transfection and cell proliferation assay
Expression and control vectors were transfected into CTLL-2 cells by electroporation, and those cells were selected by G418 (0.5 mg/mL). Two transfectants, CTLL-2/pME18Sneo and CTLL-2/sHBZ, were established. For cell proliferation studies, sHBZ-expressing CTLL-2 and the control cells were plated at a density of 1 × 104 cells/well in 96-well plates. Cells were treated with increasing concentrations of TGF-β for 72 hours and assayed for cell growth by the methyl thiazolyl tetrazolium assay. Each experiment was performed 3 times, and representative results are presented.
Retroviral constructs and transduction
sHBZ cDNA was cloned into the retroviral vectors, pGCDNsamI/N and pMXs-IG, to generate pGCDNsamI/N-sHBZ and pMXs-sHBZ-IG. Transfection of the packaging cell line, Plat-E, was performed as described.32 Mouse splenocytes were enriched for CD25−CD4+ cells with a CD4 T lymphocyte enrichment set (BD Biosciences) with the addition of biotinylated anti-CD25 antibody (BD Biosciences), and activated by antigen-presenting cells (T cell-depleted and X-irradiated (20 Gy) C57BL/6J splenocytes) in the presence of 0.5 μg/mL anti-CD3 antibody and 50 U/mL human recombinant IL-2 in 12-well plates. After 24 hours, activated T cells were transduced with viral supernatant and 4 μg/mL polybrene, and centrifuged at 1700g for 60 minutes. Cells were subsequently cultured in medium supplemented with 50 U/mL recombinant IL-2.
Lentiviral vector construction and transfection of recombinant lentivirus
We cloned sHBZ cDNA into the EcoRI site of a lentiviral vector, pCSII-EF-MCS. The IRES-NGFR cassette was inserted into the XbaI site, which is located downstream of the sHBZ gene. Lentiviral vectors were produced as described.7 Human peripheral blood mononuclear cells were enriched for CD4+CD25− cells with a human naive CD4 T-cell enrichment set (BD Biosciences) and activated by anti-CD3/28–coated beads (Invitrogen). After 24 hours, cells were incubated with concentrated vector stocks in the presence of 4 μg/mL polybrene.
Flow cytometric analysis
Cells were washed with phosphate-buffered saline containing 1% FBS. After centrifugation, cells were treated with AlexaFluor-467–conjugated anti–human NGFR antibody (BD Biosciences) for 30 minutes. Cells were fixed and permeabilized at 4°C overnight and then treated with eFluor 450 conjugated anti-Foxp3 antibody (eBioscience) at 4°C for 30 minutes. After being washed with PBS, the cells were analyzed with a flow cytometer (BD FACSCanto II, BD Biosciences).
Cell sorting
Mouse naive T cells transduced with viral supernatant derived from the pMXs-sHBZ-IG and pMXs-IG vectors were sorted for the expression of green fluorescent protein using a FACSAria with Diva software (BD Biosciences PharMingen).
Statistical analyses
Statistical analyses were performed using the unpaired Student t test.
Results
HBZ protein enhances TGF-β/Smad–mediated signaling
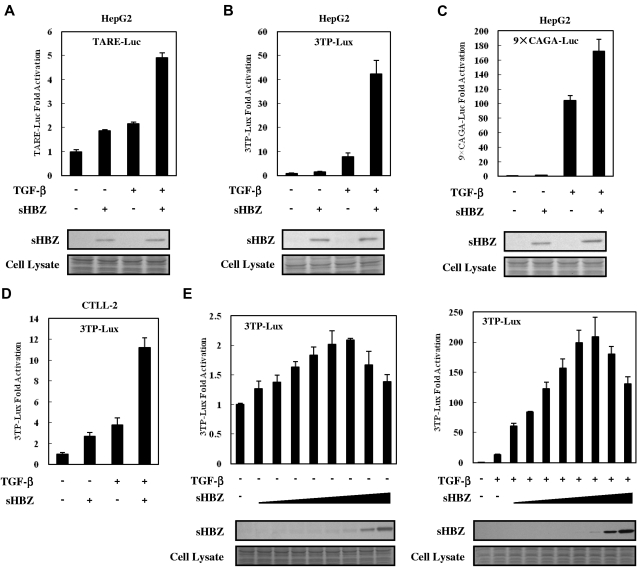
To analyze the effect of HBZ on TGF-β–mediated transcriptional responses, HepG2 cells were cotransfected with an HBZ expression vector along with different TGF-β responsive reporters: TARE-Luc, 3TP-Lux, and 9 × CAGA-Luc. As shown in Figure 1A-C, sHBZ enhanced the transcription of luciferase in each of the reporter plasmid DNAs. sHBZ also up-regulated TGF-β transcription in a mouse T-cell line, CTLL-2 (Figure 1D). TGF-β signaling was increasingly activated when less than 200 ng of sHBZ expression vector, pcDNA3.1-mycHis-sHBZ, was added to each well. At higher levels (> 200 ng) of sHBZ plasmid DNA, however, activation of TGF-β signaling partially decreased (Figure 1E). In addition, pME18Sneo-based sHBZ vector activated TGF-β signaling with the same tendency as pcDNA3.1-mycHis-sHBZ did, whereas 20 ng of pME18Sneo-sHBZ plasmid was sufficient for maximal activation (supplemental Figure 1).
Figure 1.
HBZ activated TGF-β signaling. In 12-well plates, HepG2 cells were cotransfected with 2 ng of phRL-TK, 0.5 μg of reporter plasmid TARE-Luc (A), 3TP-Lux (B), or 9 × CAGA-Luc (C), and 0.5 μg of pcDNA3.1-mycHis-sHBZ. At 24 hours after transfection, the cells were treated with TGF-β (10 ng/mL). After 24 hours, the cells were harvested and analyzed for luciferase activity. Expression of sHBZ was detected by Western blot (middle panel). Coomassie brilliant blue (CBB) staining was shown as the loading control (botom panel). (D) CTLL-2 cells were transfected with 3TP-Lux (2 μg), phRL-TK (10 ng), and pME18Sneo-sHBZ (0.4 μg) by electroporation. Luciferase activity was measured 24 hours after stimulation by TGF-β. (E) In 12-well plates, HepG2 cells were cotransfected with 3TP-Lux (0.5 μg), phRL-TK (2 ng), and pcDNA3.1-mycHis-sHBZ (0, 5, 10, 20, 50, 100, 200, 1000, and 4000 ng). At 24 hours after transfection, the cells were treated with or without TGF-β. After 24 hours, the cells were harvested and analyzed for luciferase activity. mycHis-sHBZ was detected by Western blot (middle panel). CBB staining was shown as the loading control (bottom panel).
HBZ interacts with Smads
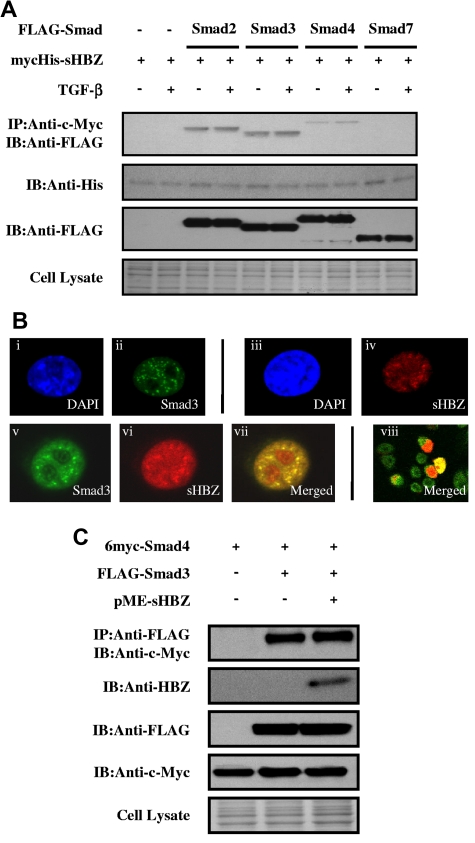
To clarify the molecular mechanism by which HBZ enhances the TGF-β transcriptional response, FLAG-tagged Smad2, Smad3, Smad4, or Smad7 and mycHis-tagged sHBZ were cotransfected into COS7 cells. Figure 2A demonstrates that sHBZ interacted with Smad2/3 and, to a lesser extent, with Smad4. TGF-β treatment had little effect on these interactions. Because a high degree of homology exists between Smad2 and Smad3, our subsequent analyses focused on the Smad3-HBZ interaction. We next examined the subcellular localization of sHBZ and Smad3 by confocal microscopy. After stimulating with TGF-β, cotransfected cells showed nuclear spots representing colocalization of sHBZ and Smad3 (Figure 2B). Activated Smad3 forms heteromeric complexes with the Co-Smad, Smad4, and translocates to the nucleus where it directly regulates transcription of target genes.15 We therefore investigated whether HBZ influenced Smad3/Smad4 complex formation. COS7 cells were transfected with 6myc-Smad4 and FLAG-Smad3 with or without sHBZ. As shown in Figure 2C, sHBZ did not influence interaction between Smad3 and Smad4.
Figure 2.
HBZ interacts with Smad proteins. (A) HBZ interacted with Smad proteins. COS7 cells were cotransfected with mycHis-sHBZ (6 μg) and FLAG-Smad2, Smad3, Smad4, and Smad7 (6 μg). At 24 hours after transfection, the cells were treated with or without TGF-β (5 ng/mL). Cell lysates were subjected to immunoprecipitation using anti-c-Myc followed by immunoblotting using anti-FLAG for detection of Smad proteins. (B) sHBZ colocalized with Smad3 in the cell nucleus. COS7 cells were transfected with mycHis-sHBZ (0.6 μg) together with (v-viii) or without (iii-iv) FLAG-Smad3 (0.4 μg). sHBZ was detected using anti-MYC Cy3 antibody (iv,vi). Smad3 was detected using anti–Flag-biotin and secondary streptavidin-Alexa-488 antibody (ii,v). The overlay of sHBZ and Smad3 is shown (vii-viii). DAPI (4,6-diamidino-2-phenylindole) was used to counterstain the nucleus (i,iii). (C) HBZ did not influence the Smad3/Smad4 interaction. COS7 cells were transfected with the indicated expression vectors (3 μg each). Cell lysates were subjected to immunoprecipitation using anti-FLAG followed by immunoblot (IB) using anti–c-Myc.
HBZ depends on the coactivator p300 to enhance TGF-β–mediated transcription
To address whether p300 is functionally required for the enhancement of TGF-β–mediated transcription by HBZ, we evaluated the effect of the adenovirus E1A oncoprotein,33 an inhibitor of p300 activity, on the ability of HBZ to enhance TGF-β transcriptional activity. As shown in Figure 3A, wild-type E1A significantly suppressed the ability of TGF-β and sHBZ to enhance transcriptional activity through Smad-responsive elements, whereas a mutant form of E1A, E1A-ΔNT, which is defective for p300/CBP binding, had no effect on these responses. Moreover, in the presence of p300, sHBZ dramatically up-regulated Smad3-mediated TGF-β activation, whereas it had a less obvious effect without p300. This synergistic effect of HBZ and p300 on TGF-β activation was augmented with TGF-β treatment (Figure 3B).
Figure 3.
HBZ activates TGF-β signaling dependent on p300. (A) E1A repressed HBZ-induced activation of TGF-β. In 12-well plates, HepG2 cells were cotransfected with 3TP-Lux (0.5 μg), phRL-TK (2 ng), pME18Sneo-sHBZ (20 ng), and pCS2+-E1A or pCS2+-E1A-ΔNT (2 ng). Luciferase activity was measured 24 hours after stimulation by TGF-β (0, 10 ng/mL). (B) HBZ synergized with Smad3 and p300 to enhance TGF-β. HepG2 cells were cotransfected with 3TP-Lux (0.5 μg), phRL-TK (2 ng), pcDNA3.1-mycHis-sHBZ (200 ng), FLAG-Smad3 (50 ng), and pCMV-p300 (2, 5 μg). At 24 hours after transfection, the cells were treated with or without TGF-β (10 ng/mL). Luciferase activity was measured after 24 hours. Expression of sHBZ, Smad3, and p300 was detected by Western blot (middle panel). CBB staining was shown as the loading control (bottom panel). (C) HBZ, Smad3, and p300 could form a ternary complex. mycHis-sHBZ (4 μg), FLAG-Smad3 (4 μg), and HA-p300 (4 μg) were cotransfected into COS7 cells, which were subsequently treated with TGF-β (5 ng/mL). Ternary complexes were detected by sequential immunoprecipitation with anti-FLAG agarose affinity gel and anti-HA antibody, followed by immunoblotting with the His antibody. (D) HBZ enhanced the interaction between Smad3 and p300. COS7 cells were cotransfected with mycHis-sHBZ (4 μg), FLAG-Smad3 (4 μg), and HA-p300 (4 μg). Cell lysates (samples from the experiment of Figure 4E) were subjected to immunoprecipitation using anti-HA followed by immunoblotting with anti-FLAG. (E) sHBZ, Smad3, and p300 bind to the Smad-responsive promoter. After transfection with mycHis-sHBZ, FLAG-Smad3, and HA-p300, and treatment with 5 ng/mL of TGF-β for 24 hours, HepG2 cells were chromatin immunoprecipitated by each indicated antibody. The precipitated DNAs and 1% of the input cell lysates were amplified by the 3TP promoter specific primers. Expression of sHBZ, Smad3, and p300 was detected by Western blot (bottom panel).
As HBZ enhanced p300 and Smad3's activation of TGF-β signaling, we next explored whether HBZ, Smad3, and p300 could form a ternary complex. The plasmid DNAs, mycHis-sHBZ, FLAG-Smad3, and HA-p300 were cotransfected into COS7 cells that were then treated with or without TGF-β. FLAG-Smad3 and its associated proteins were immunoprecipitated using anti-FLAG, eluted with FLAG peptide, and subjected to a second immunoprecipitation with anti-HA antibody. The anti-HA immunoprecipitates were then subjected to Western blot analysis with anti-His antibody. As shown in Figure 3C, we detected a specific ternary complex only when the 3 components were coexpressed, which was enhanced in the presence of TGF-β. Furthermore, sHBZ protein greatly enhanced the interaction between Smad3 and p300 (Figure 3D). To investigate the binding of sHBZ/Smad3/p300 to DNA, we performed ChIP assay in HepG2 cells that were cotransfected with 3TP-Lux reporter along with expression vectors of sHBZ, Smad3, and p300. The ChIP assay detected the association of each of 3 proteins with Smad responsive elements, which indicated that sHBZ was recruited to 3TP promoter through forming complex with Smad3/p300. Akiyoshi et al reported that c-Ski counteracted p300 and acted as a transcriptional corepressor for Smads in the TGF-β signaling pathway.34 Therefore, we studied whether HBZ could affect the interaction between Smad3 and c-Ski and found that sHBZ did not interfere with the formation of Smad3/c-Ski complex (supplemental Figure 2). These results together suggest that HBZ augments the interaction between Smad3 and p300, thereby potentiating TGF-β signaling.
Domains of HBZ responsible for enhancement of TGF-β–mediated transcription
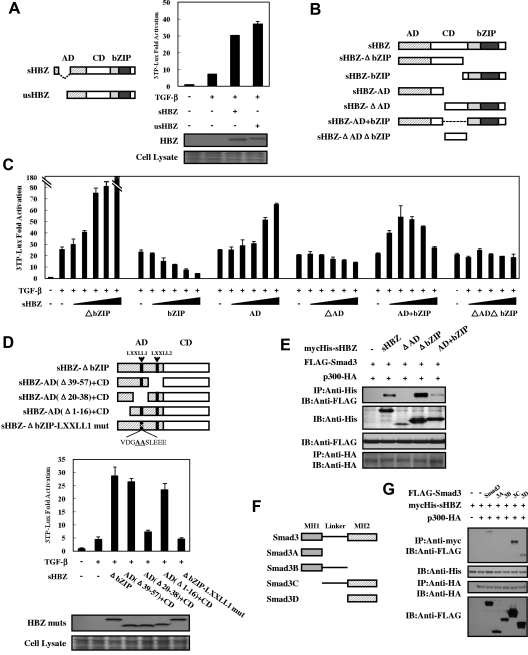
Two major isoforms of the HBZ gene have been reported: spliced (sHBZ) and unspliced (usHBZ) HBZ (Figure 4A left panel). usHBZ caused a similar activation of TGF-β responses to that caused by sHBZ (Figure 4A right panel). We next evaluated the sHBZ deletion mutants shown in Figure 4B to determine which region of HBZ is responsible for activating TGF-β signaling. Three mutants (sHBZ-AD, sHBZ-ΔbZIP, and sHBZ-AD+bZIP) enhanced the response to TGF-β, whereas the sHBZ-bZIP mutant exhibited only suppressive activity (Figure 4C). The sHBZ-ΔbZIP mutant, which maintains the central domain (CD) and the activation domain (AD), had a dramatically reinforced activation capacity compared with sHBZ-AD; however, the central domain alone (sHBZ-ΔADΔbZIP mutant) did not influence TGF-β. The sHBZ-AD+bZIP mutant displayed a similar effect to that of wild-type sHBZ (Figure 1E; Figure 4C). These results indicate that the AD domain of HBZ is responsible for the activation of TGF-β signaling whereas bZIP domain shows a suppressive effect.
Figure 4.
Domains of HBZ responsible for the activation of TGF-β signaling. (A) Comparison of the effect of sHBZ and usHBZ on TGF-β activation. (Left panel) Schematic diagram of sHBZ and usHBZ. (Right panel) HepG2 cells were cotransfected with 3TP-Lux (0.5 μg), phRL-TK (2 ng), pcDNA3.1-mycHis-sHBZ, or pcDNA3.1-mycHis-usHBZ (200 ng). Luciferase activity was measured 24 hours after 10 ng/mL TGF-β stimulation. mycHis tagged sHBZ and usHBZ were detected by Western blot (middle panel). CBB staining was shown as the loading control (bottom panel). (B) Schematic diagram of sHBZ and its mutants used in this study. Characteristic domains of sHBZ are indicated as follows: AD, CD, and basic leucine zipper domain (bZIP). (C) Analysis of sHBZ deletion mutants for their effect on TGF-β–mediated signaling. In 12-well plates, HepG2 cells were cotransfected with 3TP-Lux (0.5 μg), phRL-TK (2 ng), and pME18Sneo-sHBZ mutants (0, 5, 20, 100, 200, and 500 ng). Luciferase activity was measured 24 hours after stimulation by TGF-β (10 ng/mL). (D) The N-terminal LXXLL1 motif of HBZ is important in enhancing TGF-β–induced luciferase expression. (Top panel) Schema of sHBZ-ΔbZIP and its deletion mutants. The locations of the LXXLL motifs are indicated. The mutated residues in the LXXLL1 motif are in bold and underlined. (Middle panel) In 24-well plates, HepG2 cells were cotransfected with 3TP-Lux (0.25 μg), phRL-TK (1 ng), and mycHis-sHBZ-ΔbZIP or its mutants (1 μg). At 24 hours after transfection, the cells were stimulated with or without 10 ng/mL TGF-β. Cell lysates were subjected to luciferase assay 24 hours after stimulation. sHBZ-ΔbZIP and its mutants were detected by Western blot. CBB staining was shown as the loading control (bottom panel). (E) Determination of the region of HBZ responsible for the interaction with Smad3. COS7 cells were transfected with the indicated mycHis-sHBZ mutants along with the FLAG-Smad3 and HA-p300 vectors. Cell lysates were subjected to immunoprecipitation using anti–c-Myc followed by immunoblotting using anti-FLAG. (F) Schematic drawing of Smad3 and its deletion mutants. The locations of the MH1 domain, MH2 domain, and the linker domain are indicated. (G) Mapping the region of the Smad3 protein necessary for interaction with sHBZ. COS7 cells were transfected with HA-p300, mycHis-sHBZ, and full-length or mutant FLAG-Smad3. At 48 hours after transfection, total cell lysates were subjected to immunoprecipitation using anti–c-Myc followed by IB using anti-FLAG.
To determine the precise region within the AD domain involved in transcriptional activation, we performed reporter assays using sHBZ-ΔbZIP and its mutants (Figure 4D top panel). As illustrated in Figure 4D (middle panel), deletion of residues 20 to 38, which contains an LXXLL-like motif (LXXLL1), abrogated the enhancement of TGF-β signaling, whereas removal of the second LXXLL motif (LXXLL2) had no effect. Furthermore, we found that mutation of the LXXLL1 motif resulted in complete loss of activation. Because the LXXLL1 motif of HBZ is the major region where p300 binds,35 this result further implicates p300 in the activation of TGF-β signaling by HBZ.
We mapped the region of HBZ interacting with Smad3 in more detail. As shown in Figure 4E, full-length sHBZ and 2 of its deletion mutants (sHBZ-ΔbZIP and sHBZ-AD+bZIP) associated with Smad3, wheres sHBZ-ΔAD, which lacks the AD domain, has no binding capability. sHBZ-ΔbZIP exhibited higher affinity for Smad3 than did full-length sHBZ. This result corroborates that of the luciferase assay in Figure 4C. To define which part of Smad3 binds HBZ, we performed a coimmunoprecipitation assay with Smad3 mutants in COS7 cells (Figure 4F). We found that Smad3 mutants without MH2 domain could not bind to sHBZ whereas only the MH2 domain of Smad3 physically interacted with sHBZ. Hence, the interaction with HBZ is mediated by the MH2 segment of Smad3 (Figure 4G).
Taken together, these observations demonstrate that HBZ enhances TGF-β signaling by physically associating with Smad3/p300 complexes via its AD domain.
Activation of TGF-β signaling is partially suppressed by high doses of HBZ via inhibition of AP-1
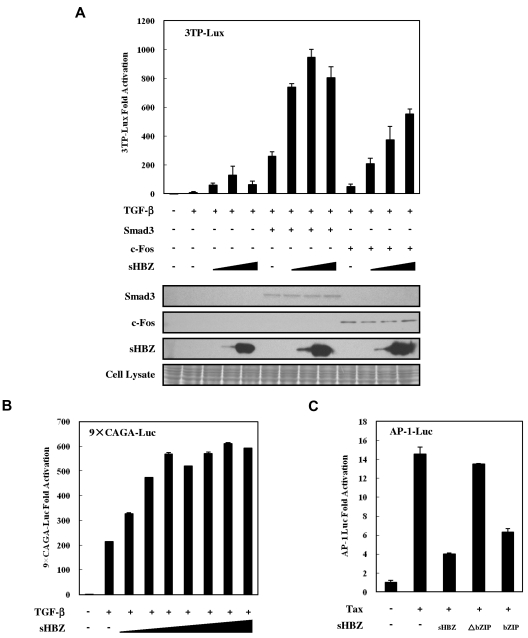
Previous studies have shown that Smads cooperate with AP-1 to mediate TGF-β–induced transcription.36 In addition, HBZ has been reported to suppress AP-1 activity.10 The observations shown in Figure 1E and Figure 4C prompted us to ask whether the activated TGF-β signaling was partially suppressed by higher HBZ expression levels because HBZ inhibited AP-1. A reporter assay showed that the suppression of TGF-β-mediated activation by high sHBZ doses (> 200 ng pcDNA3.1-mycHis-sHBZ plasmid DNA per well) was overcome by c-Fos, but not by enforced expression of Smad3, although overexpression of both Smad3 and c-Fos dramatically enhanced HBZ-mediated activation of TGF-β signaling (Figure 5A). To confirm this result, we performed a luciferase assay using a Luc vector, 9 × CAGA-Luc, which contains multiple CAGA sites but lacks AP-1 binding sites. As shown in Figure 5B, sHBZ persistently enhanced TGF-β–mediated activation of this reporter, even at high doses. In addition, we confirmed that the bZIP domain of spliced HBZ is required for the suppression of AP-1 signaling (Figure 5C). This result is consistent with the results of the luciferase assay (Figure 4C), in which sHBZ-ΔbZIP activation of TGF-β signaling was stronger than that of full-length sHBZ. These observations demonstrate that high levels of HBZ expression partially suppress TGF-β signaling by inhibiting AP-1 signaling and that the bZIP domain of HBZ is responsible for this suppressive activity.
Figure 5.
Higher expression of HBZ partially suppressed activation of TGF-β signaling via AP-1. (A) HepG2 cells were cotransfected with 3TP-Lux (0.5 μg), phRL-TK (2 ng), pCDNA3-c-Fos (0, 0.1 μg), FLAG-Smad3 (0, 0.1 μg), and pcDNA3.1-mycHis-sHBZ (0, 20, 200, and 4000 ng). At 24 hours after transfection, the cells were treated with or without TGF-β (10 ng/mL). After 24 hours, the cells were harvested, and luciferase activity was determined. Expression of sHBZ, Smad3, and c-Fos was detected by Western blot (middle panel). CBB staining was shown as the loading control (bottom panel). (B) HepG2 cells were cotransfected with 9 × CAGA-Luc (0.5 μg), phRL-TK (2 ng), and pME18Sneo-sHBZ (0, 2, 5, 10, 20, 50, 100, and 200 ng). Luciferase activity was measured 24 hours after 10 ng/mL TGF-β stimulation. (C) sHBZ inhibited AP-1 signaling via its bZIP domain. Jurkat cells were cotransfected with AP-1-Luc (1 μg), phRL-TK (10 ng), pCG-Tax (1 μg), and pME18Sneo-sHBZ mutants (1 μg). After 48 hours, luciferase activity was measured.
Physiologic levels of HBZ overcome Tax-mediated suppression of TGF-β signaling
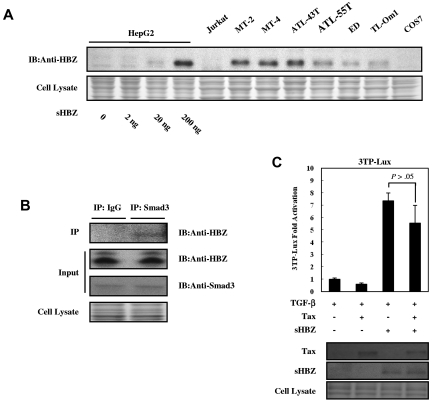
To rule out the possibility that the activated TGF-β signaling observed in this study was caused by overexpression of HBZ, we compared the expression level of HBZ protein in transfected HepG2 cells with those in ATL- and HTLV-1–associated cell lines. As shown in Figure 6A, HepG2 cells transfected with 200 ng pME18Sneo-sHBZ plasmid DNA per well on 12-well plates expressed sHBZ protein at levels equivalent or slightly higher than those in ATL- and HTLV-1–associated cell lines. This result suggested that sHBZ protein expressed at physiologic levels could therefore activate TGF-β–mediated signaling. Next, we examined the endogenous sHBZ and Smad3 protein complex. The expression of Smad3 mRNA was variable in fresh ATL cells, ATL cell lines, and HTLV-1–associated cell lines (supplemental Figure 3). sHBZ was detected in the immunoprecipitate pulled down by a specific antibody against Smad3 (Figure 6B). These data further support the interaction between HBZ and Smad3 in HTLV-1–infected cells.
Figure 6.
Physiologic level of HBZ overcame the Tax-mediated suppression of TGF-β signaling. (A) Comparing the level of HBZ protein in HBZ-transfected HepG2 cells with the level of HBZ in ATL and HTLV-1–immortalized cell lines. Total protein was extracted from sHBZ-transfected HepG2 cells (samples from supplemental Figure 1) and the indicated cell lines, and subjected to immunoblotting using HBZ antibody. (B) Endogenous HBZ interacted with Smad3. ATL-55T cells were treated with 10 ng/mL TGF-β. After 10 hours, whole cell lysate was subjected to immunoprecipitation with anti-Smad3 or control IgG, and immunoprecipitates were probed with anti-HBZ antibody. (C) HBZ overcame the repression of TGF-β signaling induced by Tax. In 12-well plates, HepG2 cells were cotransfected with 3TP-Lux (0.5 μg), phRL-TK (2 ng), pCG-Tax (0, 0.2 μg), and pcDNA3.1-mycHis-sHBZ (0, 0.2 μg). At 24 hours after transfection, the cells were treated with or without 10 ng/mL TGF-β. After 24 hours, the cells were harvested and analyzed for luciferase activity. sHBZ and Tax were detected by Western blot (middle panel). CBB staining was shown as the loading control (bottom panel).
It has been reported that HTLV-1 Tax protein inhibits Smad-dependent TGF-β signaling.18–20 Next, we performed a reporter assay to study the effect of HBZ on Tax-mediated suppression of TGF-β pathway. When coexpressed with Tax, sHBZ overcame Tax's repression of 3TP luciferase activity. Moreover, Tax had little effect on sHBZ-activated TGF-β signaling when equal amounts of sHBZ and Tax were expressed (Figure 6C).
HBZ enhances Foxp3 expression in naive T cells through Smad3
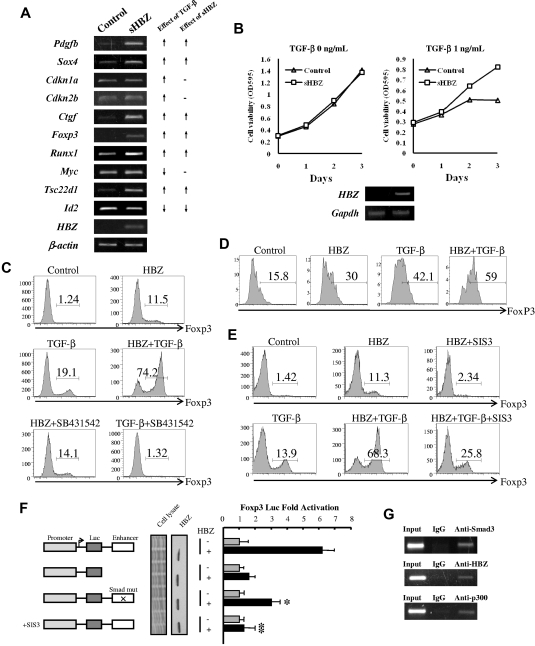
We next studied the effect of HBZ expression on transcription of TGF-β target genes. We expressed sHBZ in mouse naive T cells using a retrovirus vector. As shown in Figure 7A, expression of sHBZ was associated with enhanced transcription of Pdgfb, Sox4, Ctgf, Foxp3, Runx1, and Tsc22d1 genes and suppression of Id2 gene; such effects were consistent with those by TGF-β. However, HBZ did not influence the level of Cdkn1a, Cdkn2b, and Myc gene transcripts. This result indicates that HBZ selectively modulates transcription of TGF-β target genes, such as TGF-β. HBZ does not interfere with the expression of genes associated with cell cycle and proliferation. To check whether HBZ could affect the cell growth via enhancing TGF-β signaling, we established stable sHBZ-expressing CTLL-2 cell lines. TGF-β suppressed proliferation of control CTLL-2 cells, whereas HBZ expressing CTLL-2 cells proliferated regardless of TGF-β (Figure 7B).
Figure 7.
HBZ induced Foxp3 expression in naive T cells through Smad3. (A) HBZ modulated the expression of selected TGF-β target genes. (Left panel) Mouse naive T cells were transduced with pMXs-IG vector encoding sHBZ or empty vector. Forty-eight hours after viral infection, total RNA was extracted from sorted green fluorescent protein-positive cells. The level of Pdgfb, Sox4, Cdkn1a, Cdkn2b, Ctgf, Foxp3, Runx1, Myc, Tsc22d1, Id2, β-actin, and HBZ mRNA was analyzed by semiquantitative RT-PCR. (Right panel) Schema of the effect of TGF-β and sHBZ on TGF-β target gene transcription. ↑ indicates up-regulation; ↓, down-regulation; and -, no effect. (B) CTLL-2/pME18Sneo and CTLL-2/sHBZ, cells were plated in 96-well plates. Cells were treated with increasing concentrations of TGF-β for 72 hours. Proliferation of each cell was examined by methyl thiazolyl tetrazolium assay. Expression of HBZ was detected by RT-PCR (bottom panel). (C) SB431542, an inhibitor of the TGF-β receptor, could not inhibit the induction of Foxp3 by HBZ. Mouse CD4+CD25− T cells were transduced with pGCDNsamI/N vector encoding sHBZ, or with empty vector. Three days after TGF-β (0.2 ng/mL) and SB431542 (5μM) treatment, cells were stained with anti-Foxp3 in addition to anti-NGFR and then analyzed by flow cytometry. Numbers indicate the percentage of Foxp3-positive cells among NGFR-positive cells. (D) HBZ induced FoxP3 in human naive T cells. Human CD4+CD25− T cells were transfected with lentiviral vectors expressing sHBZ, or with empty vector. Two days after stimulating with TGF-β (0.1 ng/mL), cells were stained with antibodies for CD4, NGFR, and Foxp3 and then analyzed by flow cytometry. (E) SIS3 inhibited the HBZ-induced Foxp3 induction. Mouse CD4+CD25− T cells were transduced with pGCDNsamI/N vector encoding sHBZ or empty vector. Fifteen hours after viral infection, SIS3 (5μM) and TGF-β (1 ng/mL) were added. Thirty-six hours after treatment, Foxp3 expression was detected by flow cytometry. Numbers indicate the percentage of Foxp3-positive cells among NGFR-positive cells. Representative data from 3 independent experiments are shown. (F) HBZ activated transcription of the Foxp3 promoter through its Smad site of enhancer. EL4 cells were transfected with the Foxp3 reporter plasmid or its mutants with or without the sHBZ-expressing plasmid (pcDNA3.1-mycHis-sHBZ). Luciferase activity was measured 48 hours after stimulation by TGF-β. Expression of sHBZ was detected by Western blot. CBB staining was shown as the loading control. (G) HBZ formed complex with Smad3/p300 in FoxP3 enhancer. MT-2 cells treated with 5 ng/mL of TGF-β for 2 hours, and chromatin immunoprecipitated by each indicated antibody. The precipitated DNAs and 1% of the input cell lysates were amplified by the specific primers for FoxP3 enhancer.
It has been reported that TGF-β signaling is critical for the development of CD4+CD25+Foxp3+ regulatory T cells and that the binding of Smad3 to a specific enhancer region is required for activation of the Foxp3 promoter.21,29 As shown in Figure 7A, HBZ enhanced transcription of Foxp3 gene induced by TGF-β. We therefore studied whether HBZ has any influence on the generation of Foxp3+ T cells. Retrovirally expressed sHBZ protein increased the level of Foxp3 in conventional mouse CD4+ T cells and also synergistically enhanced TGF-β–induced Foxp3 expression (Figure 7C,E). Treatment with SB431542, an inhibitor of the TGF-β type I receptor, did not change the induction of Foxp3 by sHBZ, whereas it completely blocked the TGF-β–induced Foxp3 expression (Figure 7C), indicating that an increase in TGF-β cytokine was not involved in this synergistic effect. sHBZ also induced FoxP3 expression in human naive T cells (Figure 7D).
Apart from Smad3, STAT5 is the main factor that sustains Foxp3 expression in both Treg and effector T cells.37 Therefore, we next evaluated the effect of HBZ on STAT5 signaling. sHBZ was not capable of activating CA-STAT5a-mediated transcriptional activation of the Jγ1 promoter (supplemental Figure 4). Therefore, it is probable that HBZ induced the expression of Foxp3 through TGF-β/Smad3 responses. After treatment with SIS3, a specific inhibitor of Smad3, sHBZ-enhanced Foxp3 induction was reduced by > 70% (Figure 7E). Furthermore, the synergistic effect of sHBZ on TGF-β–induced Foxp3 expression was markedly inhibited by SIS3 (Figure 7E).
To further analyze the mechanism by which HBZ induces Foxp3 expression, we performed a reporter assay using the enhancer and promoter of the mouse Foxp3 gene. As shown in Figure 7F, sHBZ activated Foxp3-Luciferase expression but it failed to activate the Foxp3 promoter alone, indicating that the Foxp3 gene is regulated by HBZ through the enhancer. We next examined the contribution of Smad3 to HBZ-induced Foxp3 enhancer activity. Foxp3 reporter activity was reduced after mutation of the Smad-binding region of the enhancer, and treatment with SIS3 completely blocked the transactivation of Foxp3 by sHBZ (Figure 7F). Furthermore, interaction of HBZ, Smad3, and p300 to human FoxP3 enhancer was detected by ChIP assay in a HTLV-1–transformed cell line, MT-2 (Figure 7G). These results collectively indicate that the enhanced induction of Foxp3 expression by HBZ can be attributed, at least in part, to Smad3-dependent TGF-β signaling.
Discussion
Leukemic cells in most ATL cases, like Tregs, express CD4, and CD25. Foxp3 is a master regulator that controls the transcription of genes, which are critical for the suppressive function of Tregs. Two-thirds of ATL cases express FoxP3 in the tumor cells, indicating that such ATL cells are derived from Tregs.23 Indeed, it has been reported that ATL cells have a suppressive effect on bystander CD4+ T cells.38 The proportion of HTLV-1 provirus is higher in FoxP3+ Tregs than in uninfected cells.24 Thus, these findings suggest that HTLV-1 infection increases virus-infected Tregs and finally transforms them. So far, the molecular mechanisms underlying the development of ATL Tregs have not been defined. TGF-β signaling has been implicated in both the development and function of Tregs,21 and recently we reported that transgenic expression of the HBZ gene increased Tregs in vivo and HBZ enhanced transcription of the Foxp3 gene.26 Thus, HBZ induces Foxp3 expression and promotes Treg cell development in vivo. The present study links HBZ and TGF-β signaling by showing that HBZ induces Foxp3 expression via interaction with Smad3 and p300. Although HBZ impairs the suppressive function of Tregs to some extent, HBZ-expressing Tregs retain some suppressive functions.26 Indeed, the immunodeficiency observed in ATL patients and HTLV-1 carriers might be attributable to the Treg phenotype induced by HBZ. The weak immunosuppressive potential retained by HTLV-1–infected Tregs may allow them to escape host immune attack, which possibly explains why HTLV-1 favors Tregs in vivo.
Several viruses have evolved distinct strategies to modulate TGF-β signaling using their own viral proteins. Examples include hepatitis B virus pX; hepatitis C virus core protein, NS3 and NS5; Kaposi sarcoma-associated herpesvirus K-bZIP; Epstein-Barr virus LMP1; and severe acute respiratory syndrome-associated coronavirus N protein.39–44 Like HBZ, the HBV pX and severe acute respiratory syndrome N protein enhance the transcriptional responses of TGF-β. The common strategy used by viruses to modulate TGF-β signaling is the direct binding of viral proteins to Smad proteins. In this study, we demonstrated that the enhancement of the p300/Smad3 interaction by HBZ is critical for HBZ-induced TGF-β activation.
TGF-β exerts growth inhibitory effects, from which cancer cells usually escape during malignant progression. Accumulating evidence shows that TGF-β act as tumor suppressor at early stages of cancer development but can promote tumor progression at later stages of oncogenesis through tumor-cell-autonomous and host-tumor interactions. Like other cancer cells, HTLV-1–infected T cells are resistant to the growth-inhibitory effect of TGF-β.45 As a mechanism of this resistance, Tax has been shown to inhibit TGF-β–mediated signaling, resulting in escape from the suppressive effect of TGF-β.18–20 It is possible that Tax protein inhibits the growth suppressive effect of TGF-β in the early stage of ATL, whereas other mechanisms, which include increased expression of Smad7,46 and aberrant expression of MEL1S,27 suppress cytostatic effects of TGF-β/Smad signaling pathway when Tax expression is lost in the late stage. However, as shown in this study, HBZ-expressing CTLL-2 cells could proliferate in the presence of TGF-β. There are 2 possible scenarios. HBZ selectively modulates actions of TGF-β/Smad signaling pathway as shown in this study, which possibly does not influence transcription of TGF-β target genes associated with cell cycle and proliferation. Alternatively, because HBZ has growth-promoting activity,7 it might counteract cytostatic activity of TGF-β, whereas other activities of TGF-β/Smad signaling pathway are enhanced by HBZ. It has been reported that tumors use the TGF-β/Smad system to induce gene responses that promote tumor growth, invasion, evasion of immune surveillance, and metastasis.47 In HTLV-1, an important function of activated TGF-β/Smad pathway by HBZ is up-regulation of the Foxp3 gene transcription and induction of Tregs. Further studies on TGF-β signaling in ATL are necessary to clarify its roles.
Dysregulation of TGF-β signaling has been reported in HTLV-1–associated HAM/TSP.48 Expressions of TGF-β receptor II and Smad7 were suppressed in T cells of HAM/TSP patients, suggesting that these abnormalities are implicated in perturbed immune tolerance. As shown in Figure 6C, activation of TGF-β signaling by HBZ is more predominant than the suppressive effect by Tax, which might account for why more than half of ATL cases express FoxP3,23 and the proportion of FoxP3+ Tregs is higher in Tax+ T cells derived from HTLV-1 carriers and HAM/TSP patients.24 However, Smad7 expression is increased in ATL cells, indicating that TGF-β signaling is different between leukemic and carrier state. To elucidate the entire picture of TGF-β signaling in HTLV-1 infection and ATL needs further studies.
It has been reported that HBZ suppresses viral transcription from the HTLV-1 long terminal repeat by disturbing the interaction between Tax and p300/CBP, thereby displacing p300 from the viral promoter.35 However, our report showed that the binding of HBZ with p300 stabilized the Smad3-p300 complex, resulting in TGF-β activation. HBZ competes with Tax for the same domain of p300, whereas HBZ and Smad3 have different p300 binding sites. We speculate that HBZ activation or repression of p300 transcriptional activation is dependent on the p300 binding partner. It is also probable that the function of the HBZ-p300 complex depends on the capacity of HBZ to recruit p300 onto the DNA element, a bimodal effect similar to that previously reported for the Tax-p300/CBP complex.49 Consistent with our findings, a recent study reported that HBZ activated transcription of DKK1 gene by interacting with the cellular coactivators p300 and CBP.50
In conclusion, we showed that HBZ enhanced TGF-β signaling by physically interacting with Smad3/p300, leading to the up-regulation of TGF-β target genes, including Foxp3. HTLV-1 may take advantage of this mechanism to elude host immune attack, allowing the infected cells to proliferate in vivo.
Supplementary Material
Acknowledgments
This work was supported by the Ministry of Education, Science, Sports, and Culture of Japan (Scientific Research Grant-in-aid), the Novartis Foundation (M.M.), and the National Institutes of Health (CA77556 and CA100730, P.L.G.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.Z., Y.S., T.I., and M.M. designed the research; T.Z., Y.S., K.S., and P.M. performed the research; P.L.G. and T.I. provided vital reagents; T.Z., Y.S., and M.M. analyzed the data; and T.Z., Y.S., P.L.G., T.I., and M.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Masao Matsuoka, Laboratory of Virus Control, Institute for Virus Research, Kyoto University, 53 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: mmatsuok@virus.kyoto-u.ac.jp.
References
- 1.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50(3):481–492. [PubMed] [Google Scholar]
- 2.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicot C, Harrod RL, Ciminale V, Franchini G. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene. 2005;24(39):6026–6034. doi: 10.1038/sj.onc.1208977. [DOI] [PubMed] [Google Scholar]
- 4.Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24(39):5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- 5.Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7(4):270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 6.Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol. 2002;76(24):12813–12822. doi: 10.1128/JVI.76.24.12813-12822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A. 2006;103(3):720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basbous J, Arpin C, Gaudray G, Piechaczyk M, Devaux C, Mesnard JM. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-Jun and modulates their transcriptional activity. J Biol Chem. 2003;278(44):43620–43627. doi: 10.1074/jbc.M307275200. [DOI] [PubMed] [Google Scholar]
- 9.Lemasson I, Lewis MR, Polakowski N, et al. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J Virol. 2007;81(4):1543–1553. doi: 10.1128/JVI.00480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto J, Ohshima T, Isono O, Shimotohno K. HTLV-1 HBZ suppresses AP-1 activity by impairing both the DNA-binding ability and the stability of c-Jun protein. Oncogene. 2005;24(6):1001–1010. doi: 10.1038/sj.onc.1208297. [DOI] [PubMed] [Google Scholar]
- 11.Zhao T, Yasunaga J, Satou Y, et al. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-kappaB. Blood. 2009;113(12):2755–2764. doi: 10.1182/blood-2008-06-161729. [DOI] [PubMed] [Google Scholar]
- 12.Fan J, Ma G, Nosaka K, et al. APOBEC3G generates nonsense mutations in HTLV-1 proviral genomes in vivo. J Virol. 2010;84(14):7278–7287. doi: 10.1128/JVI.02239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 14.Janknecht R, Wells NJ, Hunter T. TGF-beta-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes Dev. 1998;12(14):2114–2119. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Kehrl JH, Burton J, et al. Transactivation of the transforming growth factor beta 1 (TGF-beta 1) gene by human T lymphotropic virus type 1 tax: a potential mechanism for the increased production of TGF-beta 1 in adult T cell leukemia. J Exp Med. 1990;172(1):121–129. doi: 10.1084/jem.172.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niitsu Y, Urushizaki Y, Koshida Y, et al. Expression of TGF-beta gene in adult T cell leukemia. Blood. 1988;71(1):263–266. [PubMed] [Google Scholar]
- 18.Arnulf B, Villemain A, Nicot C, et al. Human T-cell lymphotropic virus oncoprotein Tax represses TGF-beta 1 signaling in human T cells via c-Jun activation: a potential mechanism of HTLV-I leukemogenesis. Blood. 2002;100(12):4129–4138. doi: 10.1182/blood-2001-12-0372. [DOI] [PubMed] [Google Scholar]
- 19.Lee DK, Kim BC, Brady JN, Jeang KT, Kim SJ. Human T-cell lymphotropic virus type 1 tax inhibits transforming growth factor-beta signaling by blocking the association of Smad proteins with Smad-binding element. J Biol Chem. 2002;277(37):33766–33775. doi: 10.1074/jbc.M200150200. [DOI] [PubMed] [Google Scholar]
- 20.Mori N, Morishita M, Tsukazaki T, et al. Human T-cell leukemia virus type I oncoprotein Tax represses Smad-dependent transforming growth factor beta signaling through interaction with CREB-binding protein/p300. Blood. 2001;97(7):2137–2144. doi: 10.1182/blood.v97.7.2137. [DOI] [PubMed] [Google Scholar]
- 21.Bommireddy R, Doetschman T. TGFbeta1 and Tregs: alliance for tolerance. Trends Mol Med. 2007;13(11):492–501. doi: 10.1016/j.molmed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6(8):745–751. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Karube K, Ohshima K, Tsuchiya T, et al. Expression of FoxP3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004;126(1):81–84. doi: 10.1111/j.1365-2141.2004.04999.x. [DOI] [PubMed] [Google Scholar]
- 24.Toulza F, Heaps A, Tanaka Y, Taylor GP, Bangham CR. High frequency of CD4+FoxP3+ cells in HTLV-1 infection: inverse correlation with HTLV-1-specific CTL response. Blood. 2008;111(10):5047–5053. doi: 10.1182/blood-2007-10-118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamano Y, Takenouchi N, Li HC, et al. Virus-induced dysfunction of CD4+CD25+ T cells in patients with HTLV-I-associated neuroimmunological disease. J Clin Invest. 2005;115(5):1361–1368. doi: 10.1172/JCI23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satou Y, Yasunaga J, Zhao T, et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011;7(2):e1001274. doi: 10.1371/journal.ppat.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida M, Nosaka K, Yasunaga J, Nishikata I, Morishita K, Matsuoka M. Aberrant expression of the MEL1S gene identified in association with hypomethylation in adult T-cell leukemia cells. Blood. 2004;103(7):2753–2760. doi: 10.1182/blood-2003-07-2482. [DOI] [PubMed] [Google Scholar]
- 28.Kahata K, Hayashi M, Asaka M, et al. Regulation of transforming growth factor-beta and bone morphogenetic protein signalling by transcriptional coactivator GCN5. Genes Cells. 2004;9(2):143–151. doi: 10.1111/j.1365-2443.2004.00706.x. [DOI] [PubMed] [Google Scholar]
- 29.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9(2):194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 30.Arnold J, Yamamoto B, Li M, et al. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood. 2006;107(10):3976–3982. doi: 10.1182/blood-2005-11-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood. 2008;112(9):3788–3797. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7(12):1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 33.Miyagishi M, Fujii R, Hatta M, et al. Regulation of Lef-mediated transcription and p53-dependent pathway by associating beta-catenin with CBP/p300. J Biol Chem. 2000;275(45):35170–35175. doi: 10.1074/jbc.C000258200. [DOI] [PubMed] [Google Scholar]
- 34.Akiyoshi S, Inoue H, Hanai J, et al. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. J Biol Chem. 1999;274(49):35269–35277. doi: 10.1074/jbc.274.49.35269. [DOI] [PubMed] [Google Scholar]
- 35.Clerc I, Polakowski N, Andre-Arpin C, et al. An interaction between the human T cell leukemia virus type 1 basic leucine zipper factor (HBZ) and the KIX domain of p300/CBP contributes to the down-regulation of tax-dependent viral transcription by HBZ. J Biol Chem. 2008;283(35):23903–23913. doi: 10.1074/jbc.M803116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394(6696):909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- 37.Passerini L, Allan SE, Battaglia M, et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25- effector T cells. Int Immunol. 2008;20(3):421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Ishii N, Ine S, et al. Regulatory T cell-like activity of Foxp3+ adult T cell leukemia cells. Int Immunol. 2006;18(2):269–277. doi: 10.1093/intimm/dxh366. [DOI] [PubMed] [Google Scholar]
- 39.Cheng PL, Chang MH, Chao CH, Lee YH. Hepatitis C viral proteins interact with Smad3 and differentially regulate TGF-beta/Smad3-mediated transcriptional activation. Oncogene. 2004;23(47):7821–7838. doi: 10.1038/sj.onc.1208066. [DOI] [PubMed] [Google Scholar]
- 40.Choi SH, Hwang SB. Modulation of the transforming growth factor-beta signal transduction pathway by hepatitis C virus nonstructural 5A protein. J Biol Chem. 2006;281(11):7468–7478. doi: 10.1074/jbc.M512438200. [DOI] [PubMed] [Google Scholar]
- 41.Lee DK, Park SH, Yi Y, et al. The hepatitis B virus encoded oncoprotein pX amplifies TGF-beta family signaling through direct interaction with Smad4: potential mechanism of hepatitis B virus-induced liver fibrosis. Genes Dev. 2001;15(4):455–466. doi: 10.1101/gad.856201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prokova V, Mosialos G, Kardassis D. Inhibition of transforming growth factor beta signaling and Smad-dependent activation of transcription by the Latent Membrane Protein 1 of Epstein-Barr virus. J Biol Chem. 2002;277(11):9342–9350. doi: 10.1074/jbc.M109099200. [DOI] [PubMed] [Google Scholar]
- 43.Tomita M, Choe J, Tsukazaki T, Mori N. The Kaposi's sarcoma-associated herpesvirus K-bZIP protein represses transforming growth factor beta signaling through interaction with CREB-binding protein. Oncogene. 2004;23(50):8272–8281. doi: 10.1038/sj.onc.1208059. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X, Nicholls JM, Chen YG. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J Biol Chem. 2008;283(6):3272–3280. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollsberg P, Ausubel LJ, Hafler DA. Human T cell lymphotropic virus type I-induced T cell activation: resistance to TGF-beta 1-induced suppression. J Immunol. 1994;153(2):566–573. [PubMed] [Google Scholar]
- 46.Nakahata S, Yamazaki S, Nakauchi H, Morishita K. Downregulation of ZEB1 and overexpression of Smad7 contribute to resistance to TGF-beta1-mediated growth suppression in adult T-cell leukemia/lymphoma. Oncogene. 2010;29(29):4157–4169. doi: 10.1038/onc.2010.172. [DOI] [PubMed] [Google Scholar]
- 47.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6(7):506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 48.Grant C, Oh U, Yao K, Yamano Y, Jacobson S. Dysregulation of TGF-beta signaling and regulatory and effector T-cell function in virus-induced neuroinflammatory disease. Blood. 2008;111(12):5601–5609. doi: 10.1182/blood-2007-11-123430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki T, Uchida-Toita M, Yoshida M. Tax protein of HTLV-1 inhibits CBP/p300-mediated transcription by interfering with recruitment of CBP/p300 onto DNA element of E-box or p53 binding site. Oncogene. 1999;18(28):4137–4143. doi: 10.1038/sj.onc.1202766. [DOI] [PubMed] [Google Scholar]
- 50.Polakowski N, Gregory H, Mesnard JM, Lemasson I. Expression of a protein involved in bone resorption, Dkk1, is activated by HTLV-1 bZIP factor through its activation domain. Retrovirology. 2010;7:61. doi: 10.1186/1742-4690-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.