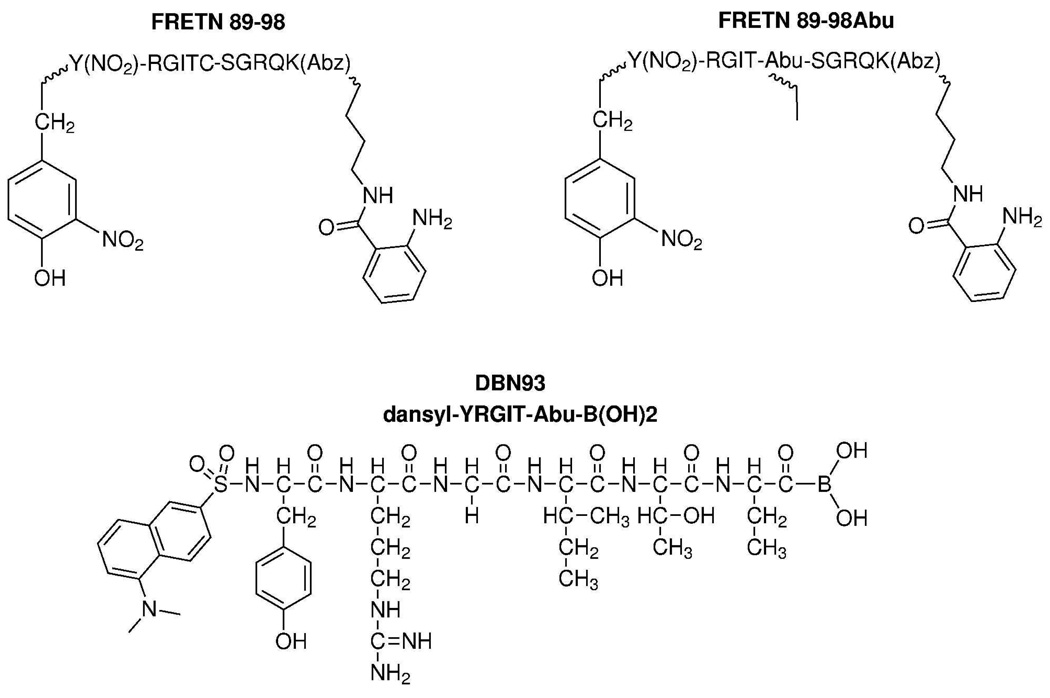
Figure 1. Structures of peptide-based substrates and inhibitor used in these experiments.
FRETN 89–98 contains an anthranilamide fluorescent donor at the carboxy terminus and a nitrotyrosine quencher at the amino terminus. Upon cleavage of the peptide at Cys-Ser, the donor and quencher separate and an increase in fluorescence emission can be monitored over time. FRETN 89–98Abu contains a substitution of Abu for Cys at the cleavage site. Inhibitor DBN93 was designed from a product of FRETN 89–98 peptide hydrolysis by Lon with a boronic acid moiety on the carboxy end to interact with the active site of the protease.