Abstract
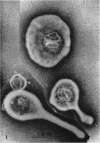
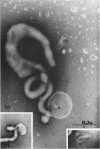
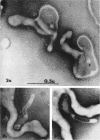
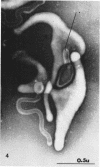
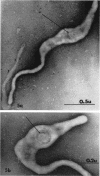
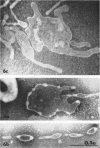
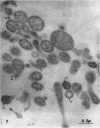
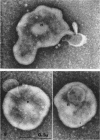
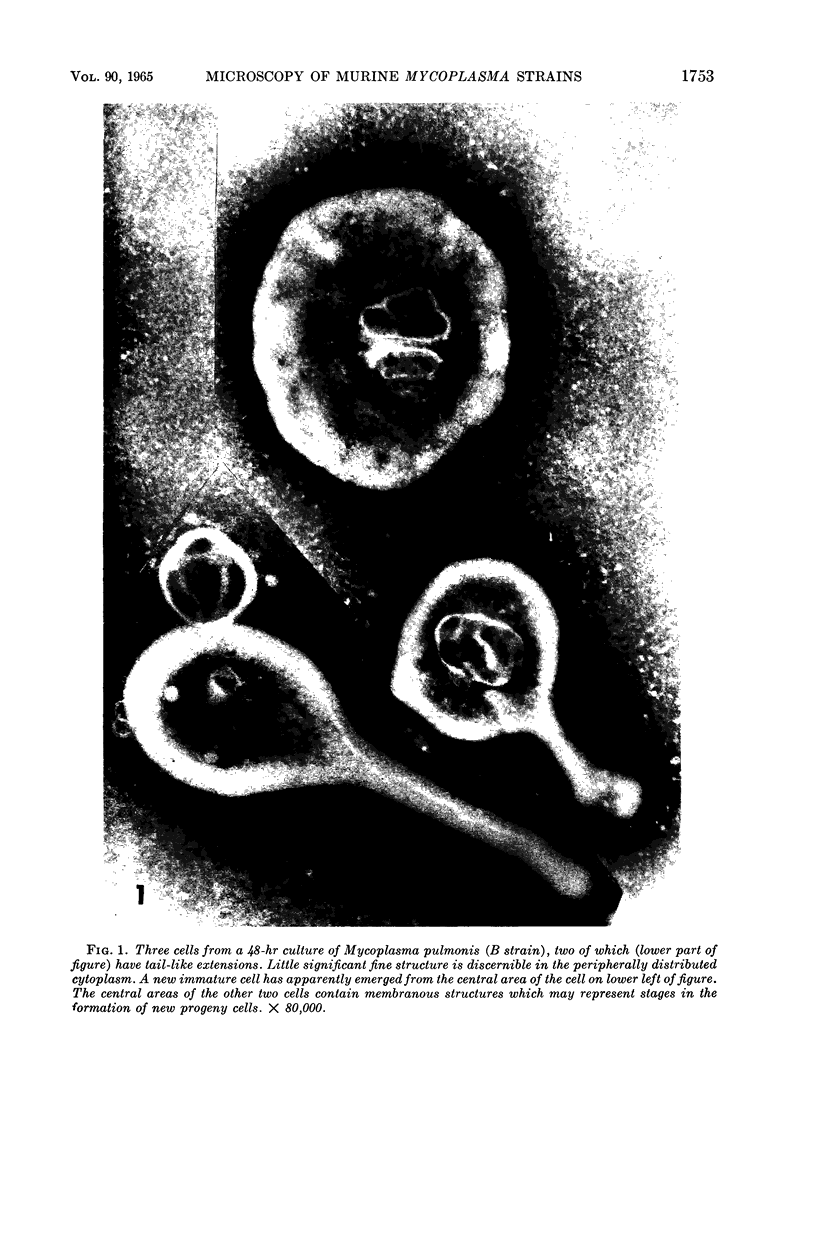
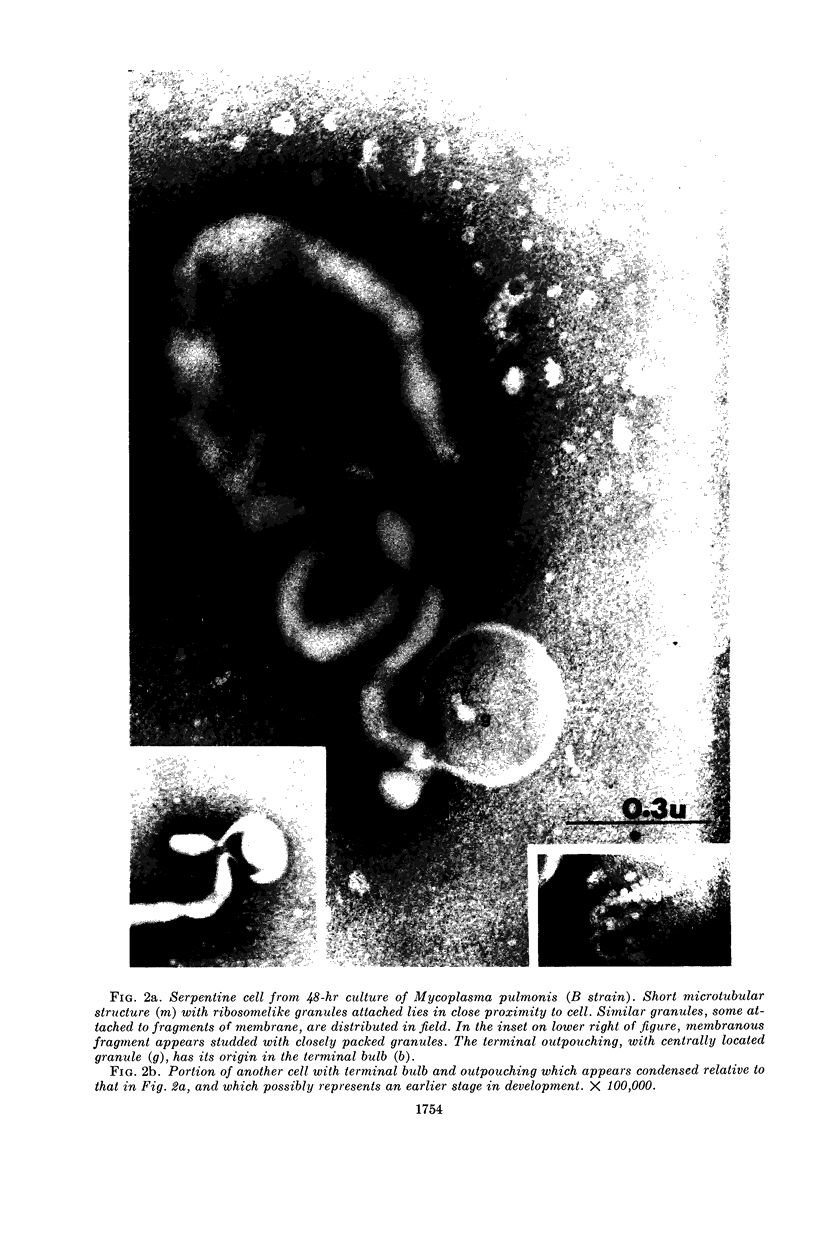
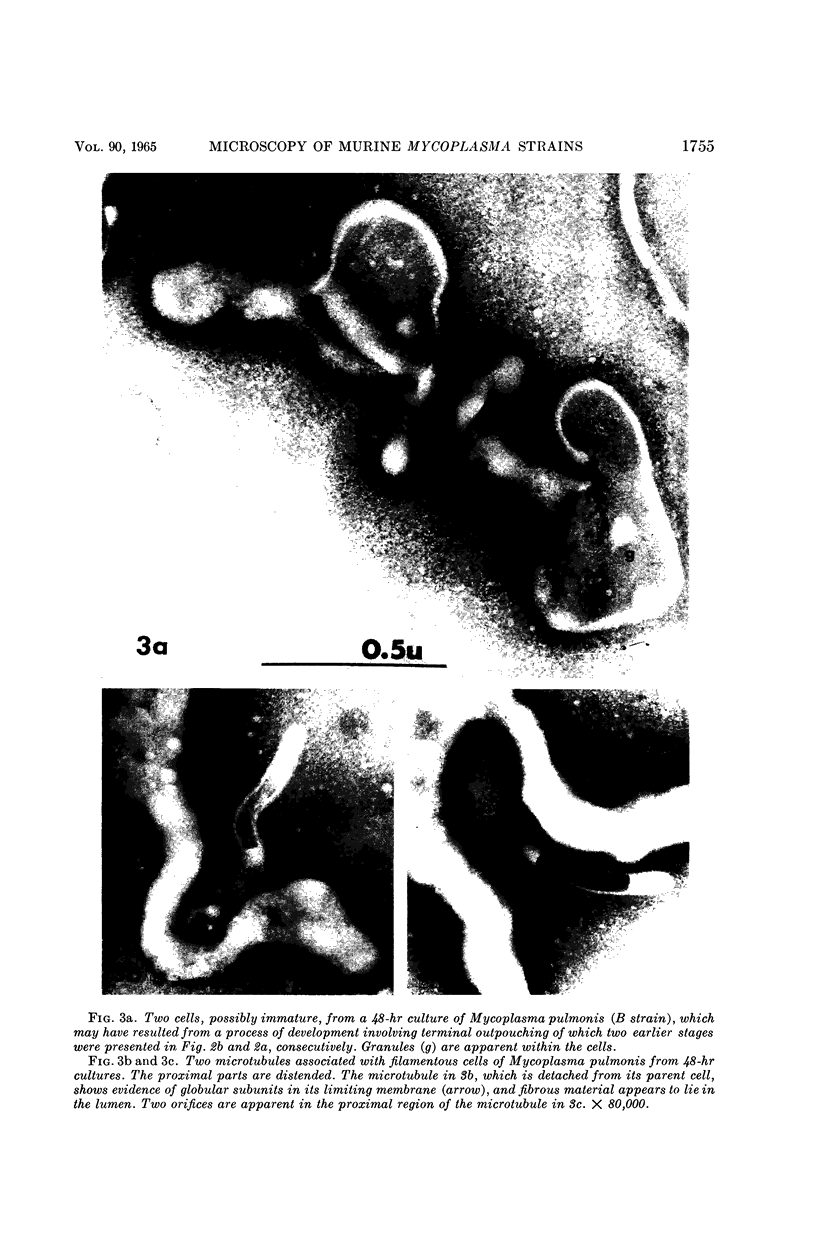
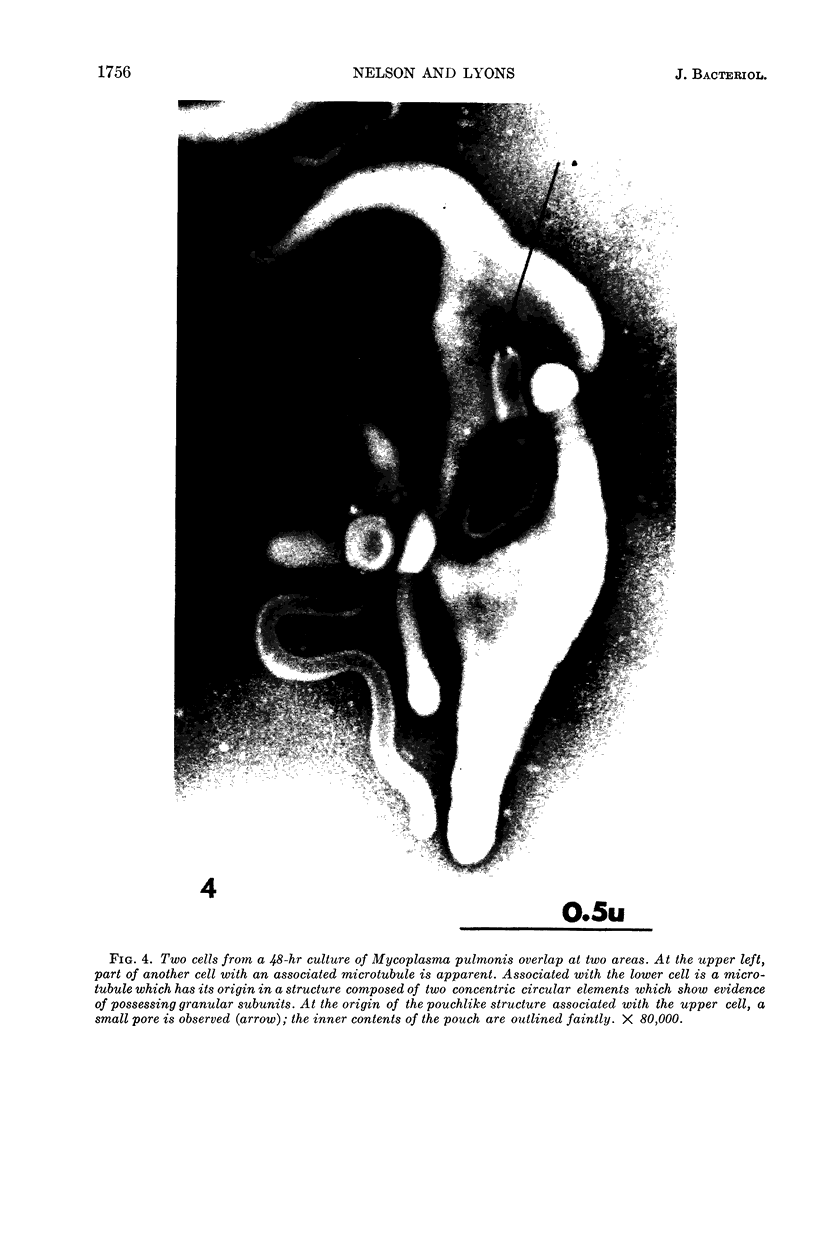
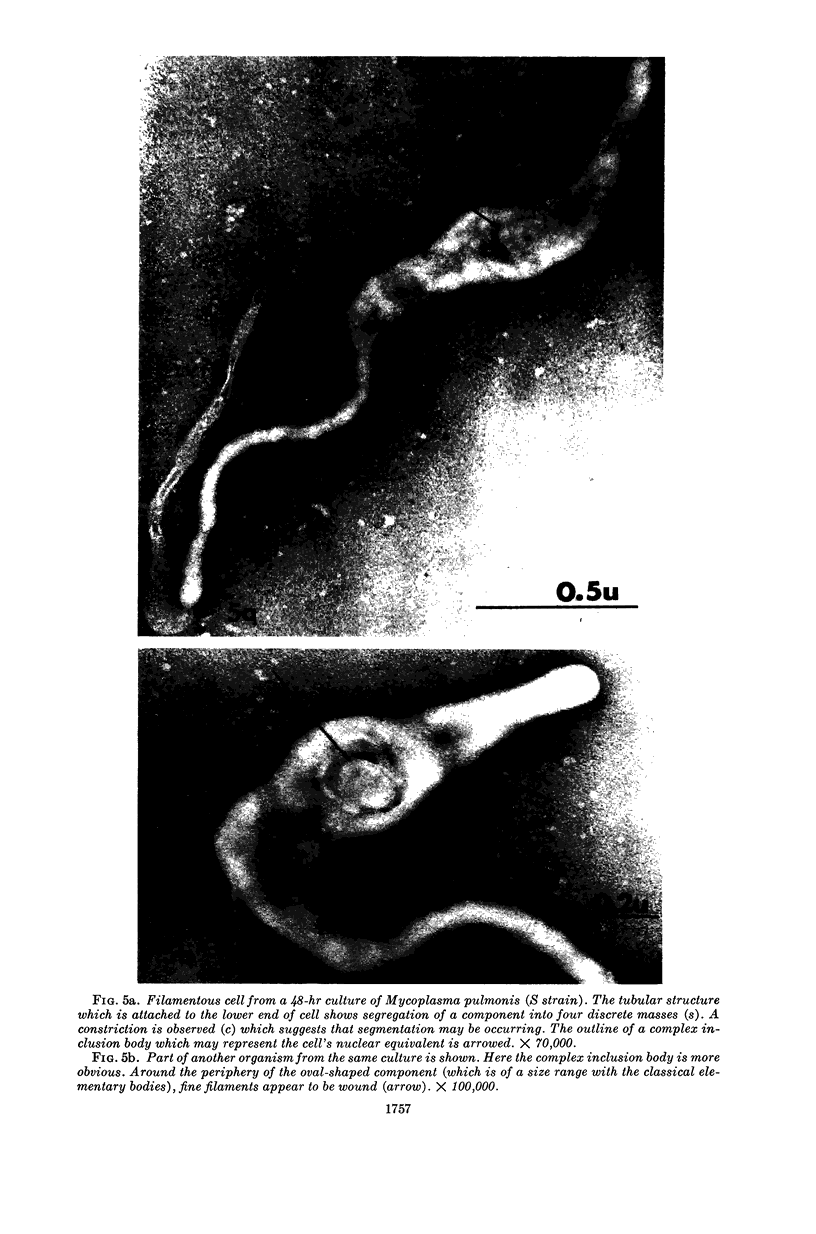
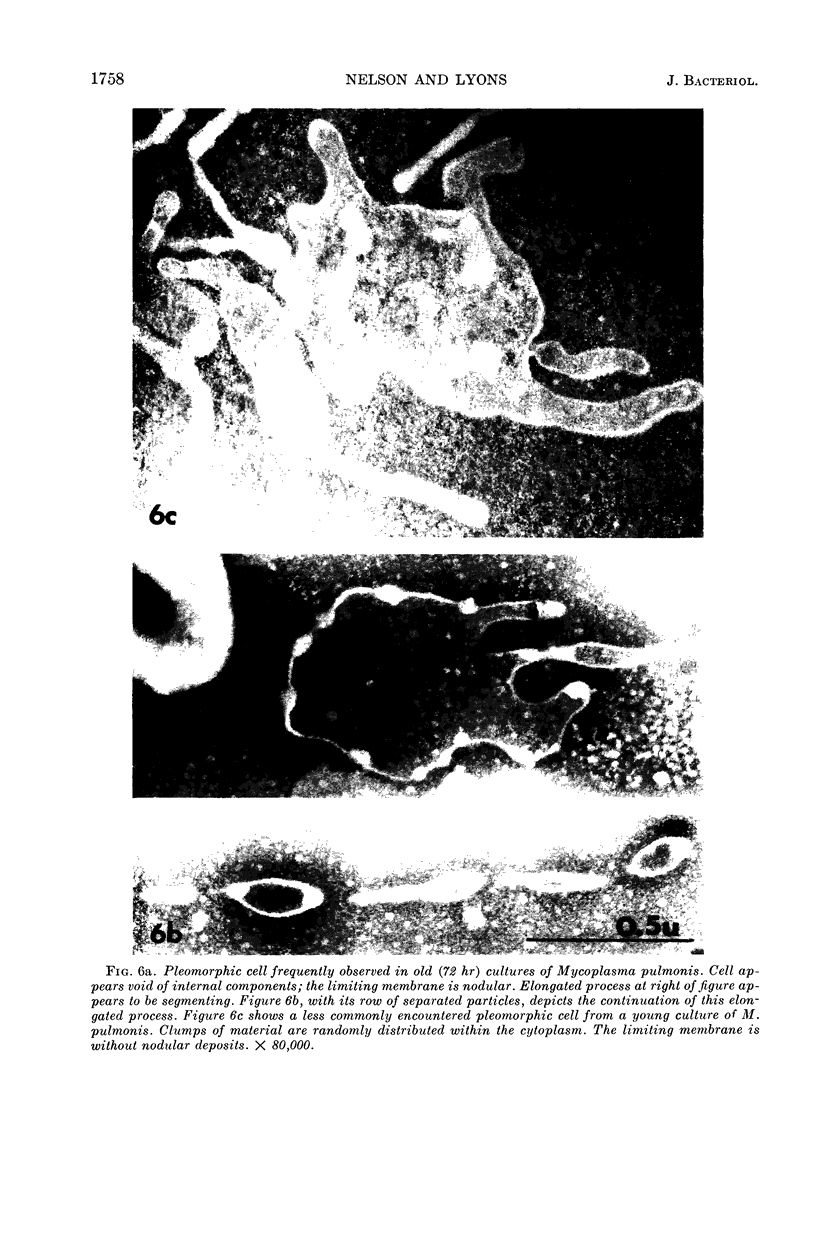
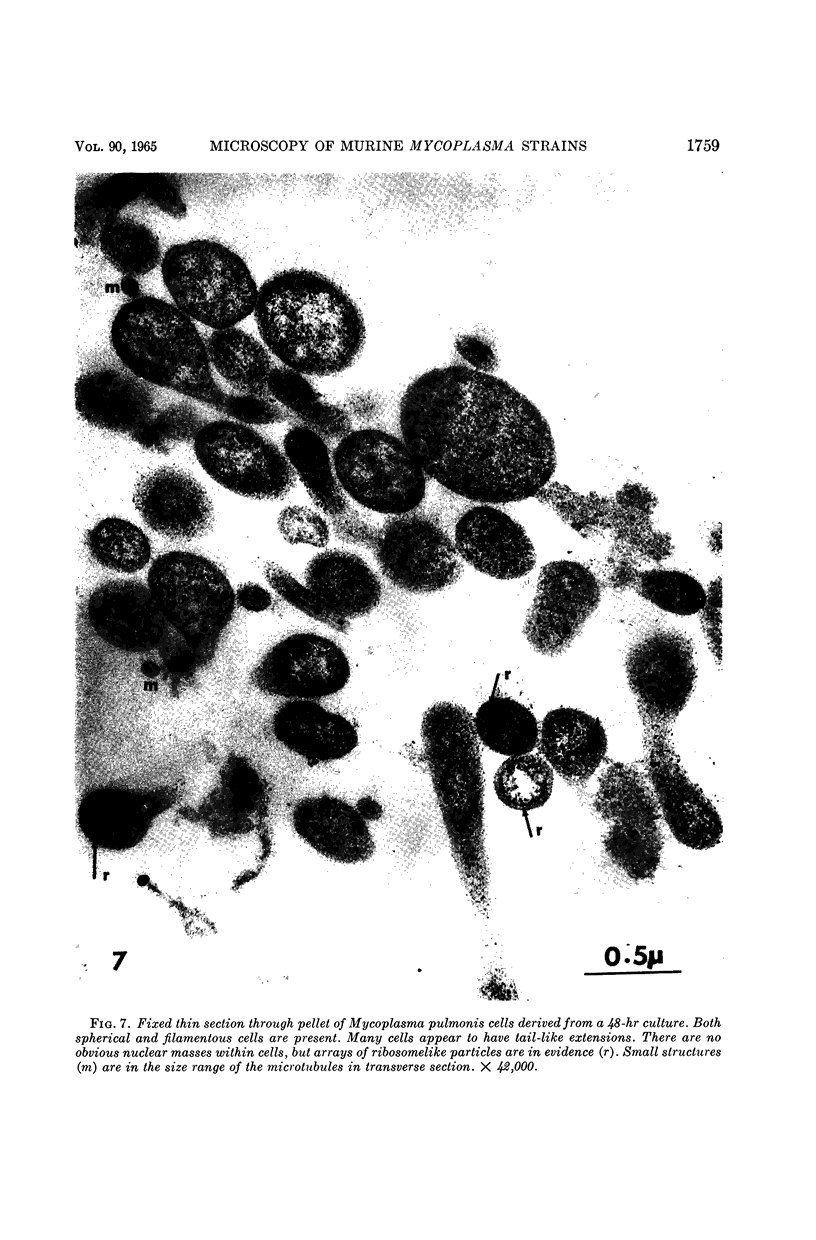
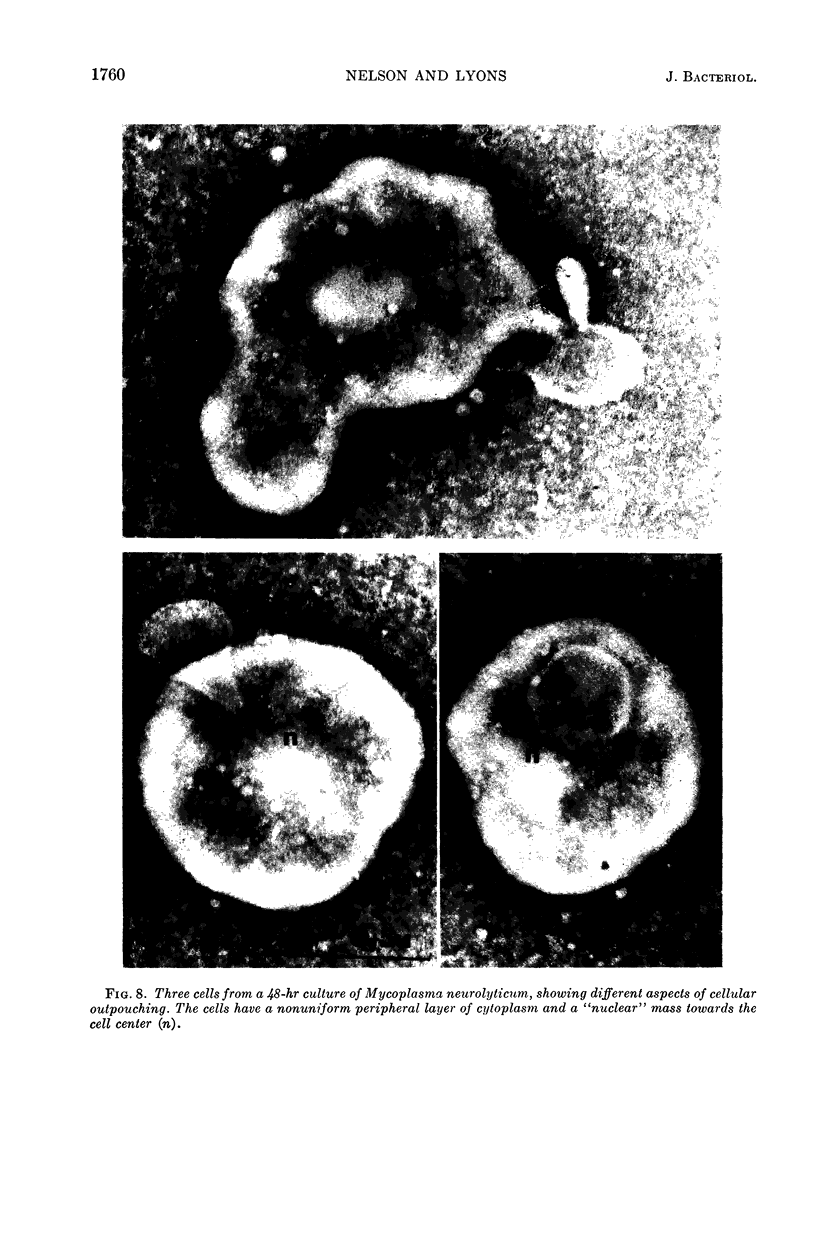
Nelson, John B. (The Rockefeller University, New York, N.Y.), and Michael J. Lyons. Phase-contrast and electron microscopy of murine strains of Mycoplasma. J. Bacteriol. 90:1750–1763. 1965.—Two strains of Mycoplasma pulmonis (associated with infectious catarrh) on examination in fluid culture (20% horse serum-bouillon) by phase microscopy were highly pleomorphic, with many bacilliform elements and fewer coccoid ones. Motility, characterized by gliding of rods and spinning of spherical forms, was observed through the 9th subculture of one strain and the 15th of the second. Motile elements were not seen in later transfers and pleomorphism was reduced. One strain of M. neurolyticum (associated with conjunctivitis and encephalitis) was much less pleomorphic and showed neither bacilliform elements nor motility at any time. When examined by negative-contrast electron microscopy, organisms of this strain were found to have an average diameter of 0.7 μ and to possess a concentrated peripheral layer of cytoplasm and a central mass which may represent the cells' nuclear equivalent. The latter feature was not prominent in spherical forms of M. pulmonis. These cells, when observed after 48 hr of culture, showed evidence of the generation of new progeny cells in their central area. The filamentous or bacilliform cells of M. pulmonis were frequently serpentine in appearance, 2.0 to 3.0 μ in length and 80 to 250 mμ in width. They appeared to generate new cells from terminal buds from which outpouchings initially developed. Older cells, in the stationary phase, showed evidence of undergoing multipolar germination. Microtubules, about 60 mμ wide, were found in association with most filamentous cells from 48-hr cultures; fragments of membrane, studded with closely packed ribosomelike particles, were also found. There was no evidence of flagella or any specialized structure that could account for the observed motility of the organisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. R., Barile M. F. Ultrastructure of Mycoplasma hominis. J Bacteriol. 1965 Jul;90(1):180–192. doi: 10.1128/jb.90.1.180-192.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- DOMERMUTH C. H., NIELSEN M. H., FREUNDT E. A., BIRCH-ANDERSEN A. ULTRASTRUCTURE OF MYCOPLASMA SPECIES. J Bacteriol. 1964 Sep;88:727–744. doi: 10.1128/jb.88.3.727-744.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G. A., FOGH J. Fine structure of pleuropneumonia-like organisms in pure culture and in infected tissue culture cells. J Bacteriol. 1960 Feb;79:267–276. doi: 10.1128/jb.79.2.267-276.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDT E. A. Morphology and classification of the PPLO. Ann N Y Acad Sci. 1960 Jan 15;79:312–325. doi: 10.1111/j.1749-6632.1960.tb42693.x. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., GLAUERT A. M. STRUCTURE AND ASSEMBLY OF MACROMOLECULAR LIPID COMPLEXES COMPOSED OF GLOBULAR MICELLES. J Mol Biol. 1964 May;8:727–748. doi: 10.1016/s0022-2836(64)80121-2. [DOI] [PubMed] [Google Scholar]
- MANILOFF J., MOROWITZ H. J., BARRNETT R. J. STUDIES OF THE ULTRASTRUCTURE AND RIBOSOMAL ARRANGEMENTS OF THE PLEUROPNEUMONIA-LIKE ORGANISM A5969. J Cell Biol. 1965 Apr;25:139–150. doi: 10.1083/jcb.25.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUYS A. C., van ITERSON Some characteristics of pathogenic avian PPLO. Antonie Van Leeuwenhoek. 1961;27:129–138. doi: 10.1007/BF02538433. [DOI] [PubMed] [Google Scholar]
- Smith W. E., Hillier J., Mudd S. Electron Micrograph Studies of Two Strains of Pleuropneumonia-like (L) Organisms of Human Derivation. J Bacteriol. 1948 Nov;56(5):589–601. doi: 10.1128/jb.56.5.589-601.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TULLY J. G. BIOCHEMICAL, MORPHOLOGICAL, AND SEROLOGICAL CHARACTERIZATION OF MYCOPLASMA OF MURINE ORIGIN. J Infect Dis. 1965 Apr;115:171–185. doi: 10.1093/infdis/115.2.171. [DOI] [PubMed] [Google Scholar]
- Weiss L. J. Electron Micrographs of Pleuropneumonia-like Organisms. J Bacteriol. 1944 Jun;47(6):523–527. doi: 10.1128/jb.47.6.523-527.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]