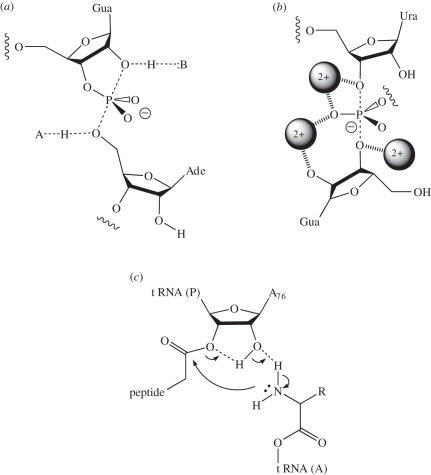
Figure 1.
Three proposed mechanisms for natural ribozymes. (a) General acid–base catalysis in the nucleolytic ribozymes. In the cleavage reaction, B acts as a general base to remove the proton from the 2′-O nucleophile, whereas A donates a proton to the 5′-oxyanion leaving group. In the ligation reaction, the roles are reversed. In the VS (shown) and hairpin ribozymes, B and A are guanine and adenine nucleobases, respectively. (b) RNA metalloenzymes. The self-splicing introns and RNase P act as metalloenzymes. Atomic substitution experiments indicate that three metal ions are bound to the transition state of the group I intron as shown. Two of the metal ion binding sites have been confirmed by crystallography, but that shown bound to the 3′-O of the exogenous guanosine has not been observed in the Azoarcus ribozyme crystal structure. (c) A possible mechanism of peptidyl transferase in the ribosome. The proposed proton shuttle between the nucleophile and the carbonyl target involving the 2′-O of the terminal A76 of the P-site tRNA is shown.