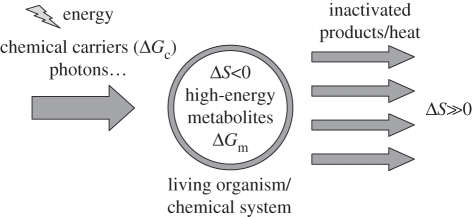
Scheme 1.
Self-organization in chemical systems induced by a flow of energy and a local decrease in entropy compensated by an overall increase. The formation of high-energy metabolites/intermediates is dependent upon the supply of energy (as chemical carriers or under physical forms) into the system and on its degradation into high entropy forms. For closed systems, at the steady state, the same amounts of energy are entering and leaving the system but energy is released in a degenerate form characterized by high entropy. Self-organization in this kind of system can depend upon the occurrence of autocatalytic pathways, self-replication processes or metabolic cycles increasing the rate of decay of high-energy reactants and constituting dissipative structures. Except unstable intermediates with very short lifetimes, the free energy potential of the metabolites accumulating in significant concentration is usually limited by the energy of chemical carriers (reactants): −ΔGm < –ΔGc, demonstrating that the incoming energy source determines the energy of proto-metabolic intermediates. Generating metabolites with higher energy potentials would require the presence of a thermodynamic machine (such as a heat engine capable of extracting work or heat from high-entropy energy forms using an entropy sink), the complexity of which is highly unlikely for primitive living forms. Therefore, the energy flow constitutes the driving force and the nature of the energy source is likely to have decisive consequences on the kind of metabolism accessible from the self-organization process.