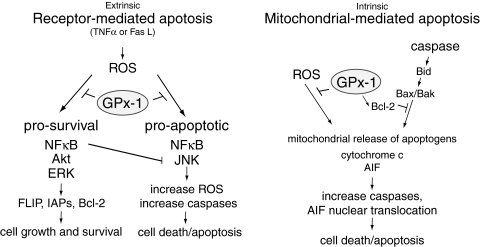
FIG. 10.
Role of GPx-1 in modulating apoptosis. The extrinsic pathway of apoptosis involves activation of caspase pathways that promote cell death. ROS activates both survival and apoptotic pathways. GPx-1 by modulating cellular hydrogen peroxide can inhibit both pro-survival and pro-apoptotic pathways. The end result (cell death or survival) may depend on the extent and levels of ROS generated. Interestingly, excess ROS as found in GPx-1 deficiency may alter nuclear factor κB (NFκB) signaling to promote pro-apoptotic responses. Normally, NFκB activation results in the upregulation of IAPs and other antiapoptotic genes. Similarly, extracellular signal-related kinase (ERK) and Akt activation can promote IAP or FLICE-inhibitory protein (FLIP) expression to inhibit caspase cascades. c-Jun-amino terminal (stress-activated) kinase (JNK) activation, which can be attenuated by NFκB, augments apoptotic pathways by further stimulating ROS production. GPx-1 overexpression has been shown to specifically suppress the activation of Akt and NFκB pathways. GPx-1 specifically blocks NFκB activation by preventing the degradation of the NFκB inhibitor inhibitor of κB. The intrinsic pathway of apoptosis involves the release of apoptogens like apoptosis-inducing factor (AIF) or cytochrome c from mitochondria. These pathways may be activated by ROS (including hydrogen and lipid hydroperoxides) and caspase cascades that promote Bid (Bcl-2 interacting domain) cleavage. GPx-1 has been shown to attenuate AIF release and enhance the expression of Bcl-2, an antiapoptotic factor.