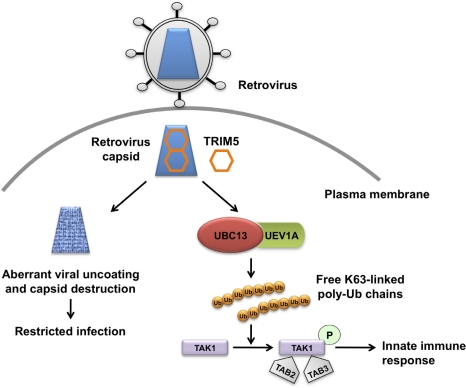
Figure 1.
The dual role of TRIM5α in retrovirus infection. The retrovirus capsid is engaged by TRIM5α in the cytoplasm of the infected cell and forms a hexagonal lattice on top of the capsid. This leads to aberrant uncoating of the capsid and blocks retrovirus infection. Concomitantly, TRIM5α binding to capsid triggers its E3 ligase activity, and in concert with the E2 ubiquitin (Ub)-conjugating enzyme complex UBC13–UEV1A generates free lysine 63 (K63)-linked Ub chains, which in turn are catalysts in the autophosphorylation (indicated as a letter P in the green circle) of the TAK1 complex (includes TAK1, TAB2, and TAB3). Activation of the TAK1 complex by autophosphorylation results in the induction and expression of NF-κB- and MAPK-responsive inflammatory genes, thereby leading to an innate immune response in the infected cell.