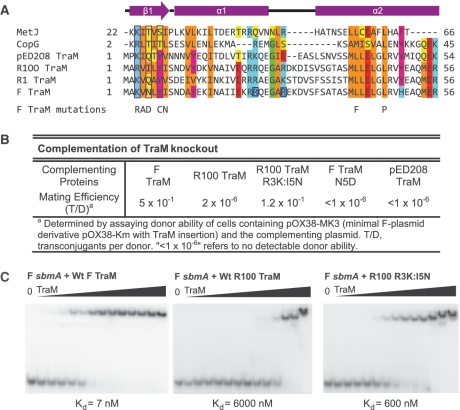
Figure 1.
The N-terminal domain of pED208 TraM binds to sbmA as a ribbon-helix-helix fold. (A) Primary structure alignment of TraM homologues and RHH fold domains. DNA-contacting β-strand residues are boxed in purple. Conserved residues are highlighted. Hydrophobic, orange; Aromatic, magenta; acidic, red; basic, cyan; Gly/Pro, green; polar aliphatic, yellow. Secondary structure elements of TraM are indicated. F TraM mutations shown in previous studies to disrupt F plasmid conjugation are indicated. R24 and R29, F TraM residues protected from trypsin digestion upon binding to sbmA, are boxed in blue. (B) Complementation of TraM proteins in a TraM-deficient F-derived plasmid system. (C) EMSA of F, R100 and R100 R3K:I5N TraM binding to 30-bp F sbmA. Concentrations in each lane are 0, 2, 5, 10, 20, 50, 100, 250, 600, 1000, 2500, 6000, 10 000 and 25 000 nM.