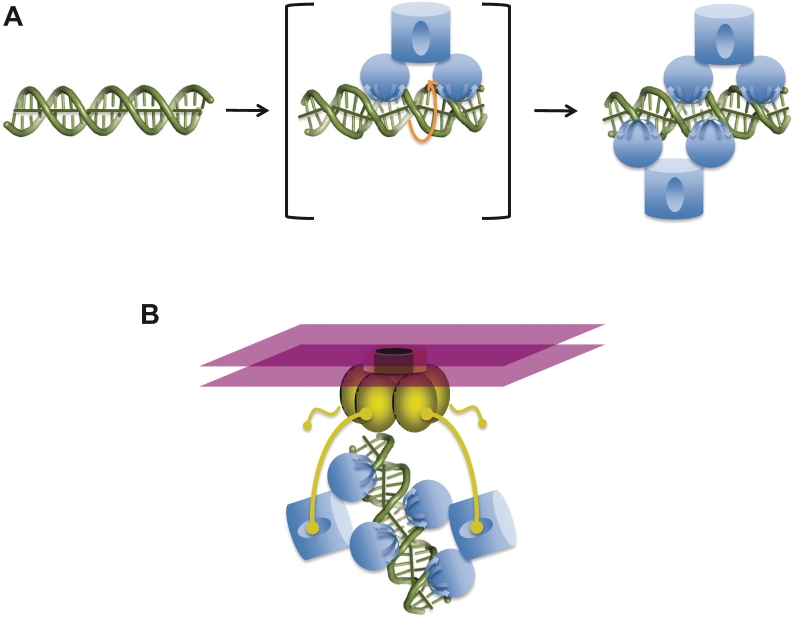
Figure 7.
Models for cooperative recognition of DNA and TraD by TraM. (A) Model for cooperative binding of sbmA by TraM. sbmA DNA exists in a B-like conformation in the absence of TraM (left). A single TraM tetramer (blue) binds a pair of GANTC elements via its two RHH domains, thereby unwinding and kinking the DNA to form an unstable intermediate complex (center panel). Binding of the first tetramer induces a DNA conformation that aligns the remaining free pair of GANTC elements on the opposite side of the DNA helix, which facilitates binding of the second tetramer and stabilization of the complex (right). (B) Stabilization of TraM–TraD interactions through an avidity effect. TraD (gold) exists as a hexamer ring complex and forms the cytoplasmic face of the conjugative pore, imbedded within the cytoplasmic membrane (purple). While isolated interactions between a single TraD tails and a TraM tetramerization domain are weak, cooperative TraM–DNA complexes provide multiple contact points for the C-terminal tails of TraD (yellow).