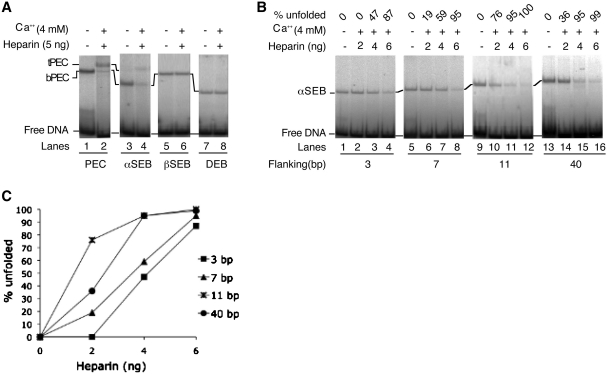
Figure 2.
Unfolding of the single end break complexes. Transpososomes were assembled by mixing appropriate combinations of un-cleaved and pre-cleaved transposon ends. Unfolding was initiated by the addition of Ca2+, an analog of the catalytic metal ion, and heparin, which sequesters any IHF that dissociates from the complex. Unfolding was analyzed using the EMSA and gels were recorded using a phosphoimager. (A) The bPEC and αSEB unfold in the presence of Ca2+ and heparin. The tPEC and the tSEB are relatively unstable during electrophoresis and the most accurate measure of unfolding is given by the disappearance of the bottom complexes (see text for details). The βSEB and the DEB are resistant to unfolding. The un-cleaved transposon end had 40 bp of flanking DNA. (B) The αSEB was assembled using un-cleaved transposon ends with progressively shorter flanking DNA. (C) Unfolding of the complexes in part B were quantified using a phosphoimager. The amount of αSEB present in the absence of Ca2+ and heparin was defined as 100%. If half of this was lost after heparin treatment, this would be given as 50% unfolding.