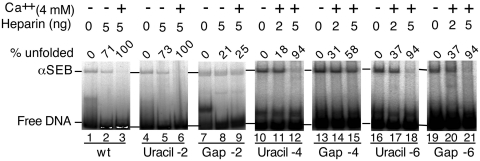
Figure 4.
Single-strand gaps in the flanking DNA inhibit unfolding. The αSEB complex was assembled from a mixture containing a pre-cleaved outside end with an un-cleaved inside end. The inside end, which is always on the β side of the transpososome, had a uracil residue in the flanking DNA at position –2, –4 or –6 of the non-transferred strand. Treatment of the uracil-containing transposon end with DNA uracil glycosylase and endonuclease VIII prior to transpososome assembly eliminated the uracil base and cleaved the phosphodiester backbone. This lesion is very similar to that caused by hydroxyl radicals in the interference assay presented in Figure 3. Unfolding was initiated by the addition of Ca2+ and heparin as previously described. Unfolding was analyzed using the EMSA and gels were recorded and quantified using a phosphoimager.