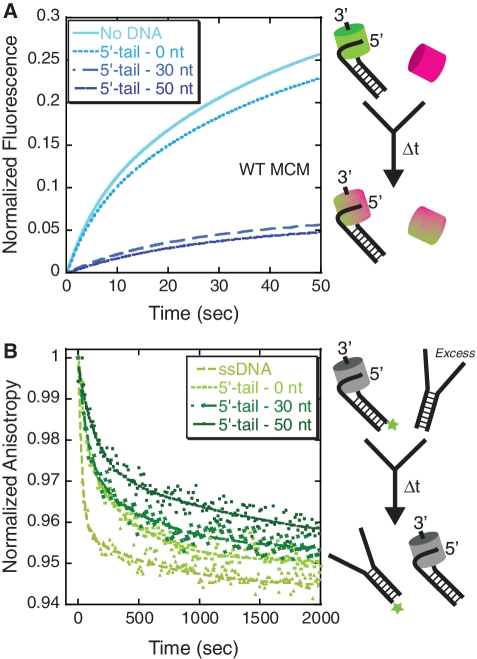
Figure 2.
The presence of a 5′-tail stabilizes the MCM hexamer. (A) Stopped flow FRET experiments were performed to detect an exchange of donor (Alexa488) and acceptor (Alexa555)-labeled MCM subunits on DNA forks with different length 5′-tails through an increase in FRET. Concentrations of SsoMCM (1.2 µM) and DNA (200 nM) were stoichiometric and were held well above the Kd value to promote the DNA-bound state. The increase in FRET was fit to two exponentials for no DNA, 5′-tail, 0 nt; 5′-tail, 30 nt; and 5′-tail, 50 nt; reported in Table 1, and attributed to the exchange of MCM subunits from solution. The cartoon shows the result of the exchange of a donor-labeled MCM bound to DNA with a free acceptor-labeled MCM complex giving rise to a mixed donor and acceptor MCM hexamer and an increase in FRET. (B) Kinetic anisotropy experiments monitoring the off-rate of the MCM complex from the fluorescently labeled DNA templates with different length 5′-tails upon addition of excess unlabeled DNA. The data was fit to Equation 2, and the individual rates are reported in Table 1. The cartoon shows the result of the dissociation of the MCM complex after trapping with unlabeled DNA leading to a decrease in the fluorescence anisotropy.