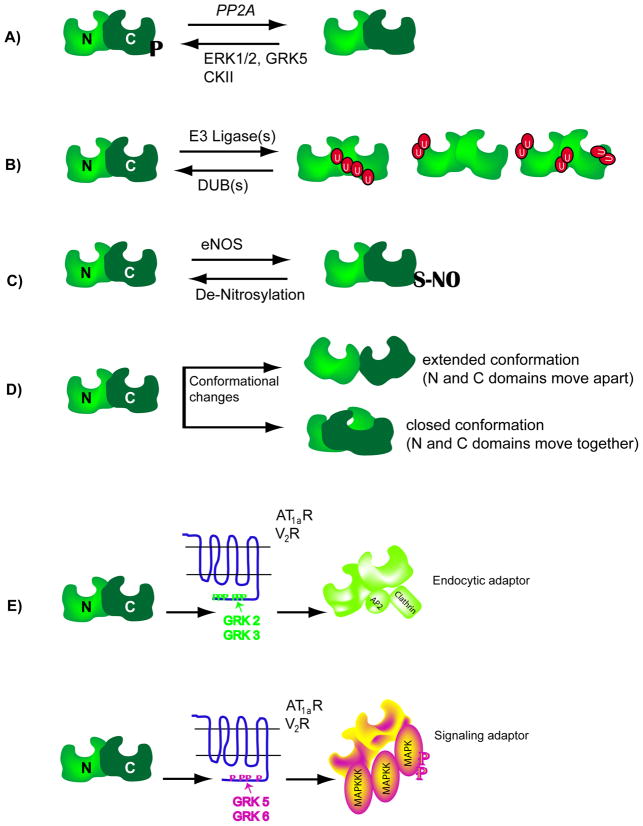
Figure 3. Schematics showing molecular changes in β-arrestin.
A) β-arrestins are phosphorylated at a seryl or threonyl residues in the C-terminal domain (see text for details) and become dephosphorylated upon 7TMR activation. Dephosphorylated β-arrestins interact more efficiently with clathrin than phosphorylated forms. Upon receptor internalization, β-arrestins become rephosphorylated. ERK1/2: extra cellular signal regulated kinase, GRK: G protein-coupled receptor kinase, CKII: casein kinase II, PP2A: protein phosphatase 2A. B) 7TMR activation leads to ubiquitination of β-arrestin2, the site of ubiquitination is unique to a particular 7TMR-β-arrestin pair, thus leading to differential modifications or differential display of ubiquitinated β-arrestins. C) The C-terminal cysteine residue in β-arrestin2 is nitrosylated and this modification promotes its interaction with clathrin. D) Upon binding to 7TMRs, the β-arrestin molecule undergoes conformational rearrangement, which could either push the amino (N) and carboxyl (C) domains apart or pull them together. The conformational changes have been assessed by various biochemical methods (limited proteolysis of purified β-arrestins, Resonance energy transfer between fluorophores attached to the two domains etc). E) When β-arrestin2 is recruited to the AT1aR or V2R that are phosphorylated by GRK2 or GRK3 isoforms, it is able to function as an endocytic adaptor, but not as a signaling adaptor. In contrast, when β-arrestin2 binds to the same receptors that are phosphorylated by GRK5 or GRK6 isoforms, it functions as a signaling adaptor leading to robust activation of MAP kinases ERK 1 and 2. It is suggested that the different GRK isoforms set respective phosphorylation ‘bar codes’ to dictate the downstream activity of β-arrestin.