Abstract
Background
Serum albumin predicts mortality in dialysis patients and is used to assess their health status and the quality of delivered care. Whether the threshold level of serum albumin at which mortality risk increases in peritoneal dialysis (PD) patients is the same as for hemodialysis (HD) patients has not been studied.
Study Design
Observational cohort study of dialysis patients undertaken to determine the survival-predictability of serum albumin in PD patients, and to compare it with that in HD patients.
Setting and Participants
130,052 dialysis patients (PD, 12,171; HD, 117,851) who received treatment in any of the 580 dialysis units owned by DaVita Inc. between 7/1/2001 through 6/30/2006, followed through 6/30/2007
Predictor
Baseline and time-averaged serum albumin (assayed by bromcresol green), and change in serum albumin over six months
Outcome Measures
All-cause, cardiovascular, and infection-related mortality.
Results
PD patients with baseline serum albumin < 3.0 g/dl had an over three-fold higher adjusted risk for all-cause and cardiovascular mortality, and 3.4-fold higher risk for infection-related mortality (reference group: serum albumin 4.00–4.19 g/dl). The adjusted all-cause mortality was significantly lower in PD patients with ≥ 0.3 g/dl increase in serum albumin over six months, and significantly higher in whom it decreased by ≥ 0.2 g/dl (reference group: serum albumin change, +0.1 to −0.1 g/dl). Significant increase in death risk was evident for HD patients with serum albumin < 4.0 g/dl but at < 3.8 g/dl for PD patients. For each albumin category, the overall death risk for PD patients was lower than of HD patients (reference group: HD patients with serum albumin, 4.00–4.19 g/dl).
Limitations
Study can only identify associations without attribution of causality and residual confounding cannot be excluded.
Conclusions
To conclude, serum albumin predicts all-cause, cardiovascular, and infection-related mortality in both PD and HD patients. However, the threshold at which risk for death increases varies by dialysis modality and this difference should be considered by agencies or organizations that set quality standards.
Keywords: peritoneal dialysis, hemodialysis, albumin, protein-energy wasting, mortality
In the United States, routine monitoring of dialysis patients invariably includes the monthly measurement of serum albumin. The presence of a low serum albumin often triggers the search for potentially reversible causes of anorexia or inflammation, and therapeutic interventions that generally consist of measures to increase the enteral protein, and energy intakes 1. Furthermore, the presence of hypoalbuminemia portends a poor short-term prognosis of a patient and is often used as a measure of the quality of delivered care. Over the years, the US Centers for Medicare and Medicaid Services has launched several initiatives to monitor the quality of care in dialysis units. Serum albumin was a key measure that was included in the Core Indicators project, which has now been replaced by the Clinical Performance Measures Project 2, 3. Even though serum albumin is not an approved clinical performance measure, it is still reported in the annual report as it “as an indicator for assessing mortality risk for adult in-center hemodialysis and peritoneal dialysis patients” 3. Indeed, many studies have demonstrated that serum albumin is a powerful predictor of outcomes in hemodialysis (HD) patients 4. In a study of 58,058 HD patients, the adjusted population-attributable fraction of death due to baseline serum albumin < 3.8 g/dl was estimated to be 19% 5. Serum albumin has been shown to predict all-cause mortality, and peritonitis risk in peritoneal dialysis (PD) patients; however, the data are significantly more limited than for HD patients 6–8. There is little, if any, information on the association of serum albumin with cause-specific mortality in PD patients. Moreover, the results reported by a handful of small studies that have evaluated the effect of change in serum albumin to outcomes in PD patients have been inconsistent. Finally, even though the serum albumin of PD patients is lower than that of HD patients – this is, in part, secondary to daily peritoneal protein losses - the adjusted five-year survival of patients receiving either PD or HD treatment is remarkably similar in different parts of the world 3, 9–11. It therefore follows that a given level of serum albumin may be associated with a lower risk for death in PD patients. If our hypothesis is correct, this would imply that the threshold of serum albumin level that mandates clinical intervention, and/or used by regulators to judge the quality of care, may differ by dialysis modality.
We undertook this study to define the relationship between serum albumin and mortality in a large, nationally representative and contemporary cohort of PD patients and to compare the relationship in PD patients to that seen in those treated with HD.
Methods
Data Source
All ESRD patients who received dialysis treatment in any of the 580 units owned by DaVita Inc between 7/1/2001 through 6/30/2006, prior to the acquisition of units owned by Gambro, constituted the study cohort. Patients were considered to be treated with PD if they were either using the therapy at the time of entry into the study cohort, or if they used the therapy at any time during follow-up. During the study period, 164,789 patients received treatment in DaVita units, data on baseline serum albumin and complete follow-up information were available for 130,052 patients (12,171 PD, and 117,851 HD); these subjects constituted the study cohort. There was no meaningful difference between any of the studied characteristics of the included and excluded patients in the entire DaVita cohort.
The presence of diabetes mellitus was ascertained using the data from DaVita. Thirteen-week averaged body weight and baseline height were used to calculate body mass index (BMI). The data of these subjects was merged with that from the United States Renal Data System (USRDS). Information on date of first dialysis treatment, race/ethnicity, marital status, insurance, and co-existing conditions was obtained from MEDVID file of the USRDS – the file contains information from Medical Evidence form 2728, a form completed at the time of first dialysis treatment for all patients in the United States. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the first day that the patient entered the cohort. The first (baseline) studied quarter for each patient was the calendar quarter in which patient’s vintage was >90 days. Ten co-morbid conditions were considered - ischemic heart disease, congestive heart failure, history of cardiac arrest, history of myocardial infarction, pericarditis, cardiac dysrhythmia, cerebrovascular events, peripheral vascular disease, chronic obstructive pulmonary disease, and cancer.
Laboratory data were obtained from DaVita Inc. All laboratory values were measured by automated and standardized methods in the DaVita laboratory (Deland, FL) within 24 hours of collection, generally on a monthly basis. Serum albumin was measured using the bromcresol green method. To minimize measurement variability, all repeated measures for each patient during the quarter when they first entered the cohort were averaged and the summary estimate was used in all models. Average values for serum albumin were obtained from up to 20 calendar quarters (q1 through q20) and used to analyze the relationship of time-averaged serum albumin with mortality. Change in serum albumin was calculated as the difference between the value six months from the time of entry into the study cohort and the baseline value.
Follow-up was available through June 30, 2007. Information on cause of death was available from the USRDS data. The study was approved as exempt by the Institutional Research Board of the Los Angeles Biomedical Research Institute at Harbor-UCLA.
Statistical Analyses
Missing covariate data were for continuous variables was imputed by the as mean or median of the existing values, as appropriate, while for categorical variables a separate group of “missing” was included in the analysis. Data on age, dialysis vintage, serum calcium, phosphorus, and alkaline phosphatase, blood hemoglobin, and white blood cell count were missing for < 1%; race, body mass index, serum creatinine, ferritin, and total iron binding capacity, and % lymphocyte count for 1–2%; primary insurance for 9%; serum parathyroid hormone for 13%; and co-morbidity for 16% of study participants. Analysis using data of only those subjects with complete information on all variables provided similar estimates (data not shown). Survival analyses including Kaplan-Meier, log-rank tests and time-dependent Cox proportional hazard regressions were used to determine the relationship of baseline, and time-averaged serum albumin, and change in serum albumin over six-months with all-cause, cardiovascular, and infection-related mortality. All analyses were “intent-to-treat” with the outcome assigned to the initial dialysis modality; subjects were censored at the time of transplant. For each analysis, three models were examined based on the level of adjustment:
Unadjusted model that included mortality data, serum albumin category, and entry calendar quarter (q1 through q20);
Case-mix adjusted model that included all of the above plus age, gender, race and ethnicity (African Americans and other self-categorized Blacks, Non-Hispanic Caucasians, Hispanics and others), and ten pre-existing co-morbid states, history of tobacco smoking, categories of dialysis vintage (<6 mos, 6 mos to 2 yrs, 2–5 yrs and ≥5 yrs), primary insurance (Medicare, Medicaid, private, and others), marital status (married, single, divorced, widowed, and other or unknown), and;
Case-mix plus malnutrition-inflammation complex syndrome (MICS) adjusted model which included all of the covariates in the case-mix model as well as BMI, and laboratory variables including serum levels of total iron-binding capacity, ferritin, creatinine, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, white blood cell count, lymphocyte percentage, and hemoglobin.
Two different sensitivity analyses were performed. In the first analysis, the relationship of baseline, and time-averaged serum albumin, and change over time was performed in the sub-group of patients who were being treated with PD at the time of entry into the cohort (n=10,528). The second sensitivity analysis was done to compare the survival predictability of serum albumin in PD and HD patients. For this, a matched cohort was built using propensity scores created using the following variables – age, gender, race/ethnicity, dialysis vintage, diabetic status, quarter of entry into the study cohort, and geographic location by state of residence (8500 pairs).
All analyses were carried out with the SAS, version 9.1 (SAS Institute, Inc., www.sas.com).
Results
Patient Characteristics
The baseline characteristics of the study cohort, stratified by dialysis modality and vintage are summarized in Table 1. PD patients were younger, less likely to be diabetic or to have Medicare as the primary insurance, and more likely to be Caucasians. They had a lower prevalence of cardiovascular co-morbidities, had higher serum creatinine, total iron binding capacity, and parathyroid hormone, and lower serum ferritin, phosphorus, and alkaline phosphatase compared to HD patients (Table 1). The same differences were seen when the patients were stratified by dialysis vintage except that among patients with vintage > 2 years, there was no difference in primary insurance for patients treated with either of the two dialysis modalities.
Table 1.
Baseline data of the study cohort, stratified by dialysis vintage.
| Variable | All patients | Vintage < 2 yrs* | Vintage ≥ 2 yrs* | |||
|---|---|---|---|---|---|---|
| PD (n=12,171) |
HD (n=117,851) |
PD (n=9,730) |
HD (n=86,837) |
PD (n=2,441) |
HD (n=31,014) |
|
| Age (years) | 54 ± 16 | 62 ± 16 | 55 ± 17 | 63 ± 16 | 52 ± 15 | 59 ± 15 |
| Age >65 years | 29 | 46 | 31 | 49 | 22 | 40 |
| Women | 47 | 45 | 46 | 45 | 51 | 46 |
| Diabetes mellitus | 38 | 45 | 41 | 47 | 26 | 38 |
| Race and/or ethnicity | ||||||
| Caucasians | 54 | 43 | 56 | 46 | 43 | 34 |
| Blacks | 23 | 32 | 20 | 29 | 31 | 41 |
| Hispanics | 13 | 15 | 13 | 15 | 14 | 14 |
| Others | 10 | 10 | 10 | 9 | 12 | 11 |
| Dialysis Vintage (mo) | 3 (1, 17) | 3 (1, 26) | 2 (1, 3) | 2 (1, 4) | 46 (33, 72) | 50 (35, 78) |
| Primary insurance | ||||||
| Medicare | 58 | 68 | 53 | 63 | 81 | 81 |
| Medicaid | 4 | 6 | 4 | 7 | 4 | 6 |
| Others | 38 | 26 | 43 | 30 | 15 | 13 |
| Co-morbidities | ||||||
| IHD | 15 | 19 | 16 | 21 | 10 | 16 |
| CHF | 18 | 27 | 19 | 29 | 14 | 23 |
| MI | 6 | 7 | 6 | 7 | 4 | 5 |
| Cardiac arrest | 0.5 | 0.6 | 0.5 | 0.6 | 0.3 | 0.5 |
| Pericarditis | 0.6 | 0.6 | 0.5 | 0.6 | 0.9 | 0.9 |
| Cardiac dysrhythmia | 3 | 4 | 3 | 5 | 3 | 3 |
| CBVD | 5 | 7 | 5 | 8 | 4 | 6 |
| PVD | 8 | 11 | 9 | 12 | 6 | 9 |
| COPD | 4 | 6 | 4 | 6 | 2 | 4 |
| Current Smokers | 5 | 5 | 5 | 5 | 6 | 5 |
| Weight (kg) | 76 ± 20 | 75 ± 21 | 76 ± 20 | 75 ± 21 | 78 ± 22 | 74 ± 21 |
| BMI (kg/m2) | 27 ± 8 | 27 ± 7 | 27 ± 8 | 27 ± 7 | 27 ± 6 | 26 ± 7 |
| Serum albumin (g/dL) | 3.6 ± 0.5 | 3.7 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.8 ± 0.4 |
| Creatinine (mg/dL) | 8.4 ± 3.8 | 8.0 ± 3.3 | 7.8 ± 3.5 | 7.2 ± 3.1 | 11.2 ± 4.0 | 10.0 ± 3.2 |
| Ferritin (ng/mL) | 245 (112, 498) | 388 (183, 720) | 223 (103, 441) | 314 (155, 595) | 394 (168, 748) | 656 (382, 973) |
| TIBC (mg/dL) | 237 ± 51 | 208 ± 47 | 240 ± 50 | 212 ± 48 | 225 ± 51 | 194 ± 41 |
| Calcium (mg/dL) | 9.2 ± 0.8 | 9.2 ± 0.7 | 9.2 ± 0.7 | 9.1 ± 0.7 | 9.3 ± 0.9 | 9.3 ± 0.8 |
| Phosphorus (mg/dL) | 5.4 ± 1.4 | 5.6 ± 1.5 | 5.3 ± 1.4 | 5.5 ± 1.5 | 5.6 ± 1.6 | 5.8 ± 1.6 |
| PTH (pg/ml) | 283 (157, 514) | 247 (144, 422) | 271 (154, 475) | 235 (138, 385) | 345 (170, 691) | 291 (162, 556) |
| Alkaline Phosphatase (U/L) | 115 ± 85 | 121 ± 93 | 113 ± 80 | 117 ± 90 | 125 ± 100 | 130 ± 101 |
| Hemoglobin (g/dL) | 12.1 ± 1.5 | 12.0 ± 1.4 | 12.2 ± 1.5 | 12.0 ± 1.4 | 11.7 ± 1.6 | 11.8 ± 1.3 |
| WBC count (x103/µl) | 7.6 ± 2.6 | 7.5 ± 2.6 | 7.6 ± 2.5 | 7.7 ± 2.7 | 7.6 ± 3.0 | 7.0 ± 2.4 |
| % lymphocyte | 20 ± 8 | 20 ± 8 | 20 ± 8 | 20 ± 8 | 20 ± 8 | 21 ± 8 |
Categorical variables are given as percentage; continuous variables as mean +/− SD or median (25th, 75th percentile).
Data on dialysis vintage missing on 13,238 HD patients and 507 PD patients
COPD, chronic obstructive pulmonary disease; CHF, Congestive heart failure; IHD, ischemic heart disease; MI, myocardial infarction; PVD, peripheral vascular disease; BMI, body mass index; PTH, parathyroid hormone; CBVD, cerebrovascular disease; PD, peritoneal dialysis; HD, hemodialysis; TIBC, total iron-binding capacity; WBC, white blood cells
Conversion factors for units: albumin and hemoglobin in g/dL to g/L, x10; creatinine in mg/dL to umol/L, x88.4; calcium in mg/dL to mmol/L, x0.2495; phosphorus in mg/dL to mmol/L, x0.3229.
No conversion necessary for ferritin in ng/mL and ug/L, PTH in pg/mL and ng/L, and WBC count in 10^3/uL and 10^9/L.
Serum albumin and mortality in PD patients
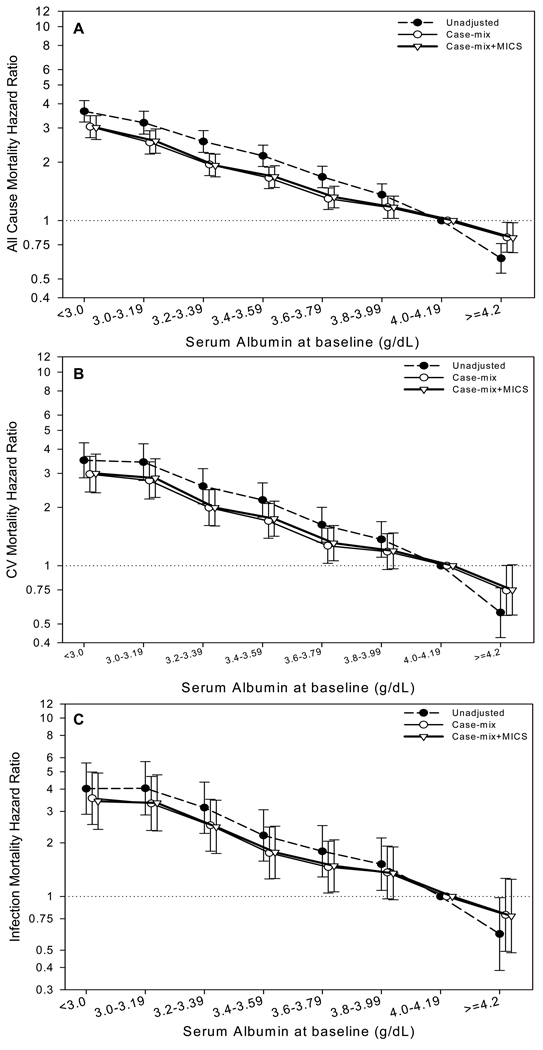
Among the 12,171 PD patients, the baseline serum albumin was < 3.0 g/dl and ≥ 4.0 g/dl in 11% and 21% of patients respectively (Table 2). Over a median follow-up of 24 months, 4,930 patients died (Table S1, available as online supplementary material). Of these, 1,876 died of cardiovascular causes and 777 of infection-related causes. There was a graded relationship between baseline serum albumin and all-cause, cardiovascular, and infection-related mortality (Tables 2 and S1, and Figure 1A, 1B, and 1C). Individuals with baseline serum albumin < 3.0 g/dl had an over three-fold higher adjusted risk for all-cause and cardiovascular mortality, and 3.4-fold higher risk for infection-related mortality (reference group comprised those with serum albumin of 4.00–4.19 g/dl). Furthermore, the adjusted risk for all-cause mortality was 18% lower in individuals with serum albumin ≥ 4.2 g/dl (serum albumin of reference group, 4.00–4.19 g/dl). The trends were similar, but accentuated, when time-averaged serum albumin was used instead of baseline serum albumin (Table 3 and Figures S1A, S1B, and S1C).
Table 2.
Association of baseline serum albumin and all-cause, CV, and infection-related mortality in PD patients
| Baseline serum albumin (g/dl) |
Proportion of population (%) |
HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|---|
| All-Cause Mortality | CV Mortality | Infection-Related Mortality | ||
| < 3.0 | 11 | 3.01 (2.62–3.47) | 2.99 (2.38–3.77) | 3.42 (2.38–4.92) |
| 3.00 – 3.19 | 8 | 2.57 (2.23–2.96) | 2.84 (2.25–3.57) | 3.35 (2.33–4.81) |
| 3.20 – 3.39 | 11 | 1.92 (1.68–2.20) | 1.99 (1.60–2.48) | 2.46 (1.74–3.46) |
| 3.40 – 3.59 | 16 | 1.68 (1.48–1.92) | 1.75 (1.42–2.15) | 1.77 (1.26–2.48) |
| 3.60 – 3.79 | 17 | 1.32 (1.16–1.50) | 1.31 (1.06–1.61) | 1.49 (1.06–2.08) |
| 3.80 – 3.99 | 16 | 1.17 (1.03–1.34) | 1.19 (0.96–1.48) | 1.35 (0.96–1.90) |
| 4.00 – 4.19 | 11 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥ 4.19 | 10 | 0.82 (0.68–0.97) | 0.75 (0.56–1.01) | 0.78 (0.48–1.25) |
Using 12,171 PD patients with serum albumin between 4.00 and 4.19 g/dl at baseline as the reference group. Data adjusted for albumin category, quarte of entry into study cohort, age, gender, race and/or ethnicity, diabetes, dialysis vintage, primary insurance, marital status, ischemic heart disease, congestive heart failure, myocardial infarction, cardiac arrest, pericarditis, cardiac dysrythmia, cerebrovascular disease, peripheral vascular disease, current smoking, body mass index, serum total iron binding capacity, ferritin, creatinine, calcium, phosphorus, parathyroid hormone, alkaline phosphatase, hemoglobin, white blood cell count, and % lymphocyte
CV, cardiovascular; HR, hazard ratio; CI, confidence interval; PD, peritoneal dialysis.
Figure 1.
Association of baseline serum albumin concentration with outcomes in patients undergoing peritoneal dialysis (n=12,171). 1A) all-cause mortality; 1B) cardiovascular mortality; and 1C) infection-related mortality. Reference group: peritoneal dialysis patients with serum albumin between 4.00 and 4.19 g/dl.
Table 3.
Association of time-averaged serum albumin and all-cause, CV, and infection-related mortality in PD patients
| Time-averaged serum albumin (g/dl) |
HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|
| All-Cause Mortality | CV Mortality | Infection-Related Mortality | |
| < 3.0 | 5.80 (4.88–6.89) | 6.23 (4.67–8.31) | 7.16 (4.60–11.15) |
| 3.00 – 3.19 | 4.40 (3.70–5.23) | 4.83 (3.62–6.44) | 5.34 (3.42–8.33) |
| 3.20 – 3.39 | 3.21 (2.72–3.79) | 3.18 (2.40–4.21) | 3.89 (2.53–5.97) |
| 3.40 – 3.59 | 2.45 (2.07–2.88) | 2.79 (2.12–3.68) | 2.45 (1.59–3.77) |
| 3.60 – 3.79 | 1.83 (1.55–2.16) | 2.07 (1.57–2.73) | 1.54 (0.98–2.39) |
| 3.80 – 3.99 | 1.37 (1.15–1.64) | 1.34 (0.99–1.81) | 1.44 (0.91–2.28) |
| 4.00 – 4.19 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥ 4.19 | 0.86 (0.63-1.17) | 1.04 (0.64–1.71) | 0.81 (0.37–1.81) |
Using 12,171 PD patients with time-averaged serum albumin between 4.00 and 4.19 g/dl as the reference group. Data adjusted for albumin category by modality, quarter of entry into study cohort, age, gender, race and/or ethnicity, diabetes, dialysis vintage, primary insurance, marital status, ischemic heart disease, congestive heart failure, myocardial infarction, cardiac arrest, pericarditis, cardiac dysrythmia, cerebrovascular disease, peripheral vascular disease, current smoking, body mass index, serum total iron binding capacity, ferritin, creatinine, calcium, phosphorus, parathyroid hormone, alkaline phosphatase, hemoglobin, white blood cell count, and % lymphocyte
CV, cardiovascular; HR, hazard ratio; CI, confidence interval; PD, peritoneal dialysis.
Change in serum albumin over six months and mortality in PD patients
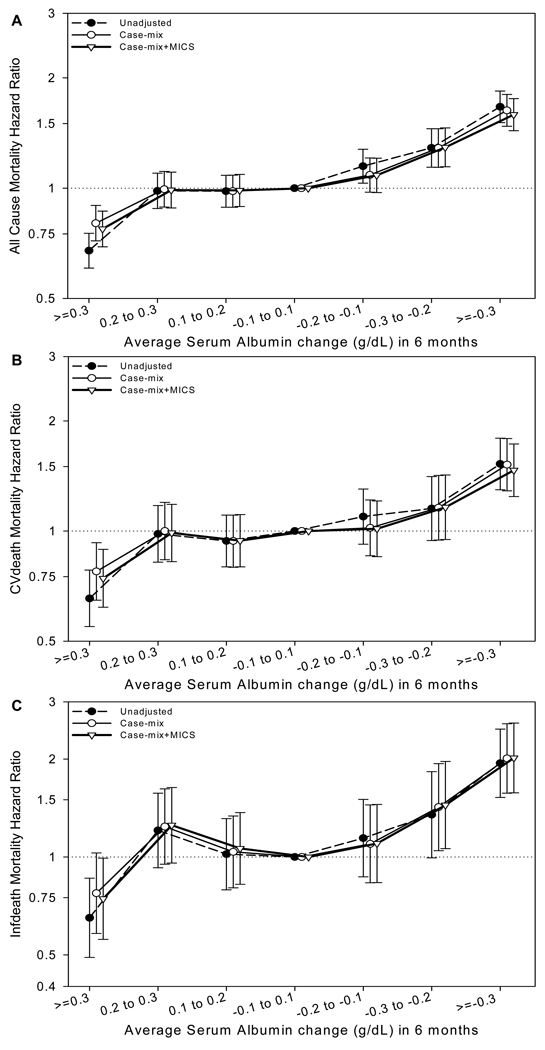
Over six months, serum albumin was unchanged (between +0.1 and −0.1 g/dl) in 3221 patients (31%), increased by ≥ 0.1 g/dl in 3777 patients (37%), and decreased by ≥ 0.1 g/dl in 3325 patients (32%). The characteristics of patients based upon the magnitude of change in serum albumin are summarized in Table 4. Patients in whom the serum albumin increased by ≥ 0.3 g/dl were younger and had the lowest serum albumin compared to other groups. Patients in whom the serum albumin decreased by ≥ 0.3 g/dl had a higher prevalence of a history of ischemic heart disease, congestive heart failure, acute myocardial infarction, and peripheral vascular disease, had lower body weight but a higher serum total iron binding capacity and parathyroid hormone compared to other groups. Furthermore, the unadjusted mortality was lowest among individuals who had ≥ 0.3 g/dl increase in serum albumin (35%) and highest among those in whom it decreased by ≥ 0.3 g/dl (46%).
Table 4.
Baseline characteristics and outcomes of PD patients categorized by the magnitude of change in serum albumin over the first six months
| Increase in serum albumin | Unchanged | Decrease in serum albumin | |||||
|---|---|---|---|---|---|---|---|
| ≥0.3 g/dL (n=1356) |
0.2–0.3 g/dL (n=1013) |
0.1–0.2 g/dL (n=1408) |
+0.1 to −0.1 g/dL (n=3221) |
0.1–0.2 g/dL (n=1177) |
0.2–0.3 g/dL (n=860) |
≥0.3 g/dL (n=1288) |
|
| Age (years) | 51 ± 17 | 54 ± 16 | 55 ± 16 | 54 ± 16 | 55 ± 16 | 54 ± 16 | 54 ± 17 |
| Age >65 years | 23 | 28 | 29 | 27 | 30 | 27 | 31 |
| women | 45 | 43 | 47 | 49 | 48 | 48 | 50 |
| Diabetes mellitus | 38 | 41 | 37 | 36 | 37 | 36 | 40 |
| Race and/or ethnicity | |||||||
| Caucasians | 47 | 52 | 52 | 51 | 53 | 53 | 55 |
| Blacks | 25 | 24 | 21 | 23 | 24 | 24 | 23 |
| Hispanics | 16 | 14 | 15 | 14 | 12 | 13 | 12 |
| Others | 12 | 10 | 12 | 12 | 12 | 10 | 10 |
| Dialysis Vintage (mos) | 2 (1, 8) | 2 (1, 10) | 3 (1, 18) | 3 (2, 23) | 3 (1, 21) | 3 (1, 20) | 2 (1, 16) |
| Primary Insurance | |||||||
| Medicare | 55 | 56 | 60 | 60 | 60 | 60 | 60 |
| Medicaid | 6 | 4 | 4 | 4 | 3 | 4 | 5 |
| Others | 39 | 41 | 37 | 36 | 37 | 36 | 35 |
| Co-Morbidities | |||||||
| IHD | 12 | 12 | 15 | 14 | 16 | 13 | 16 |
| CHF | 15 | 18 | 17 | 16 | 19 | 18 | 19 |
| MI | 5 | 4 | 4 | 6 | 6 | 6 | 7 |
| Cardiac arrest | 0.3 | 0.1 | 0.2 | 0.7 | 0.3 | 0.9 | 0.4 |
| Pericarditis | 0.8 | 0.3 | 0.2 | 0.6 | 0.5 | 0.5 | 0.4 |
| Cardiac dysrythmia | 2 | 3 | 4 | 3 | 2 | 4 | 3 |
| CBVD | 4 | 6 | 5 | 5 | 4 | 5 | 6 |
| PVD | 7 | 7 | 8 | 7 | 7 | 8 | 11 |
| COPD | 4 | 4 | 3 | 3 | 4 | 4 | 5 |
| Current smokers | 5 | 7 | 5 | 5 | 6 | 5 | 6 |
| Weight (kg) | 74 ± 19 | 79 ± 20 | 76 ± 20 | 79 ± 21 | 79 ± 20 | 77 ± 20 | 72 ± 19 |
| BMI (kg/m2) | 27 ± 7 | 27 ± 7 | 27 ± 6 | 27 ± 7 | 27 ± 8 | 27 ± 8 | 27 ± 7 |
| Serum albumin (g/dl) | 3.3 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.5 |
| Creatinine (mg/dl) | 8.4 ± 3.8 | 8.2 ± 3.6 | 8.3 ± 3.7 | 8.7 ± 3.9 | 8.8 ± 3.9 | 8.7 ± 3.9 | 8.4 ± 3.7 |
| Ferritin (ng/ml) | 270 (116, 526) |
250 (112, 483) |
227 (113, 457) |
250 (108, 511) |
233 (105, 482) |
240 (114,4 85) |
252 (116, 513) |
| TIBC (mg/dl) | 222 ± 52 | 236 ± 50 | 238 ± 48 | 240 ± 49 | 244 ± 50 | 242 ± 50 | 238 ± 48 |
| Calcium (mg/dl) | 9.0 ± 0.8 | 9.1 ± 0.7 | 9.2 ± 0.7 | 9.2 ± 0.8 | 9.3 ± 0.8 | 9.3 ± 0.8 | 9.3 ± 0.8 |
| Phosphorus (mg/dl) | 5.4 ± 1.5 | 5.3 ± 1.4 | 5.3 ± 1.4 | 5.4 ± 1.4 | 5.4 ± 1.4 | 5.4 ± 1.4 | 5.5 ± 1.5 |
| PTH (pg/ml) | 283 (162, 493) |
274 (154, 471) |
282 (150, 505) |
291 (160, 535) |
285 (159, 503) |
278 (152, 530) |
294 (162, 567) |
| Alkaline phosphatase (U/L) |
117 ± 90 | 113 ± 77 | 113 ± 82 | 115 ± 92 | 109 ± 68 | 113 ± 81 | 118 ± 81 |
| Hemoglobin (g/dl) | 11.7 ± 1.7 | 12.1 ± 1.4 | 12.1 ± 1.4 | 12.2 ± 1.5 | 12.4 ± 1.5 | 12.4 ± 1.5 | 12.2 ± 1.5 |
| WBC count (X 103/µL) | 7.7 ± 3.0 | 7.6 ± 2.3 | 7.5 ± 2.5 | 7.5 ± 2.4 | 7.7 ± 2.3 | 7.6 ± 2.4 | 7.7 ± 3.2 |
| % Lymphocyte count | 19 ± 8 | 20 ± 8 | 20 ± 8 | 20 ± 8 | 20 ± 8 | 20 ± 8 | 19 ± 8 |
| Mortality | 35 | 42 | 40 | 39 | 39 | 39 | 46 |
Categorical variables are given as percentage; continuous variables as mean +/− SD or median (25th, 75th percentile).
COPD, chronic obstructive pulmonary disease; CHF, Congestive heart failure; IHD, schemic heart disease; MI, myocardial infarction; PVD, peripheral vascular disease; BMI, body mass index; PTH, parathyroid hormone; CBVD, cerebrovascular disease; PD, peritoneal dialysis; HD, hemodialysis; TIBC, total iron-binding capacity; WBC, white blood cells
Conversion factors for units: albumin and hemoglobin in g/dL to g/L, x10; creatinine in mg/dL to umol/L, x88.4; calcium in mg/dL to mmol/L, x0.2495; phosphorus in mg/dL to mmol/L, x0.3229.
No conversion necessary for ferritin in ng/mL and ug/L, PTH in pg/mL and ng/L, and WBC count in 10^3/uL and 10^9/L
Using patients in whom the serum albumin did not change over six months as the reference group, the adjusted all-cause mortality was significantly lower among those in whom serum albumin increased by ≥ 0.3 g/dl and significantly higher in those in whom it decreased by ≥ 0.2 g/dl (Figure 2A). Similar trends were observed for cardiovascular and infection-related mortality (Figures 2B and 2C respectively).
Figure 2.
Association of change in serum albumin concentration with outcomes in patients undergoing peritoneal dialysis (n=10,323). 3A) all-cause mortality; 3B) cardiovascular mortality; and 3C) infection-related mortality. Reference group: peritoneal dialysis patients in whom serum albumin changed between +0.1 and −0.1 g/dl.
Comparison of predictive value of serum albumin in unmatched population
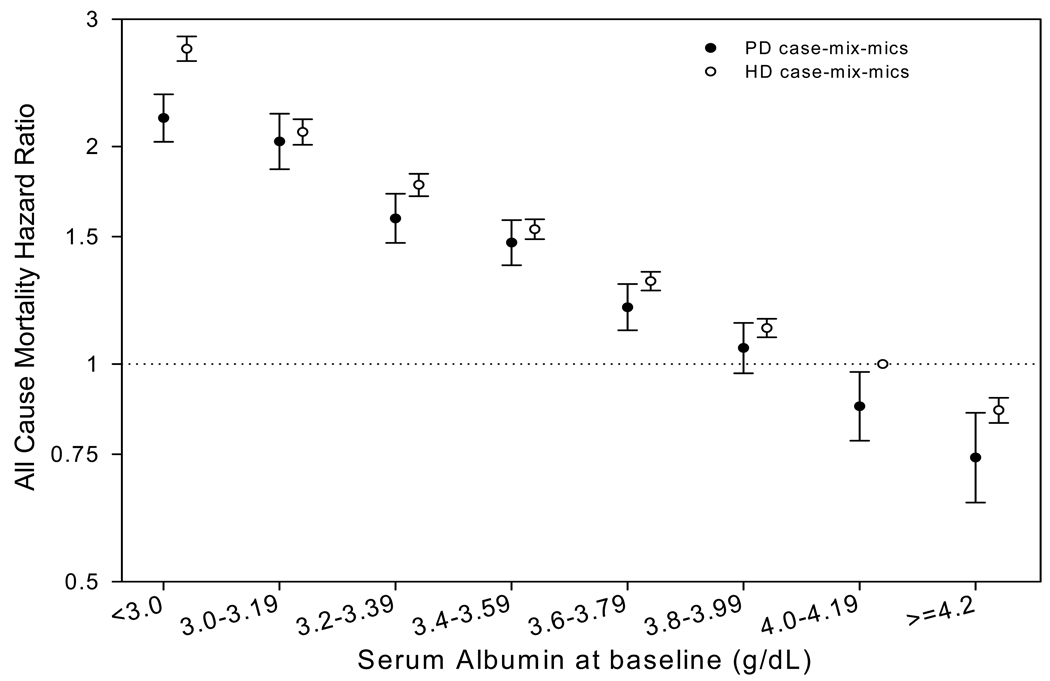
Dialysis modality was a significant effect-modifier of the relationship between serum albumin and all-cause mortality (p-value for interaction term, < 0.001). The adjusted risk for all-cause mortality for each category of baseline serum albumin for patients treated with PD and HD (reference group, HD patients with serum albumin between 4.00 and 4.19 g/dl at baseline), is summarized in Tables 5, S1, and Figure 3A. For virtually every category of serum albumin, the adjusted risk for all-cause and cardiovascular mortality for PD patients was lower than for those treated with HD. In HD patients, the risk for all-cause mortality was progressively higher for each albumin category below 4.00 g/dl; in PD patients, an increase in risk for death was seen only when the serum albumin was < 3.80 g/dl. In contrast, the risk for infection-related mortality was higher in PD patients for virtually every category of serum albumin. The same trends were seen when the data were stratified by dialysis vintage, or when time-averaged serum albumin concentrations were used (data not shown).
Table 5.
Association of baseline serum albumin and all-cause, CV, and infection-related mortality in dialysis patients
| Baseline serum albumin (g/dl) |
Proportion of population, % |
Adjusted Hazards Ratio (95% confidence interval)3 | ||||||
|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality | CV Mortality | Infection-Related Mortality | ||||||
| PD1 | HD2 | PD | HD | PD | HD | PD | HD | |
| < 3.0 | 11 | 9 | 2.19 (2.03– 2.36) |
2.73 (2.63– 2.84) |
2.16 (1.91– 2.45) |
2.32 (2.17–2.47) | 3.14 (2.58–3.82) | 4.05 (3.64– 4.51) |
| 3.00 – 3.19 |
8 | 6 | 2.03 (1.86– 2.22) |
2.09 (2.01– 2.18) |
2.19 (1.91– 2.52) |
1.99 (1.86–2.13) | 3.52 (2.84–4.36) | 2.87 (2.56– 3.21) |
| 3.20 – 3.39 |
11 | 9 | 1.59 (1.47– 1.72) |
1.77 (1.71– 1.83) |
1.57 (1.39– 1.78) |
1.68 (1.59–1.78) | 2.67 (2.21–3.24) | 2.29 (2.07– 2.53) |
| 3.40 – 3.59 |
16 | 13 | 1.47 (1.37– 1.58) |
1.54 (1.49– 1.59) |
1.46 (1.30– 1.63) |
1.51 (1.43–1.57) | 2.12 (1.75–2.57) | 1.95 (1.78– 2.13) |
| 3.60 – 3.79 |
17 | 18 | 1.20 (1.11– 1.29) |
1.30 (1.26– 1.34) |
1.12 (0.99– 1.27) |
1.29 (1.23–1.35) | 1.83 (1.51–2.21) | 1.48 (1.36– 1.62) |
| 3.80 – 3.99 |
16 | 20 | 1.05 (0.97– 1.14) |
1.12 (1.09– 1.16) |
1.02 (0.90– 1.16) |
1.12 (1.07–1.17) | 1.68 (1.37–2.07) | 1.18 (1.08– 1.29) |
| 4.00 – 4.19 |
11 | 15 | 0.87 (0.78– 0.97) |
1.00 | 0.84 (0.70– 0.99) |
1.00 | 1.25 (0.94–1.67) | 1.00 |
| ≥ 4.19 | 10 | 11 | 0.74 (0.64– 0.86) |
0.86 (0.83– 0.89) |
0.63 (0.50– 0.81) |
0.87 (0.82–0.93) | 1.04 (0.71–1.53) | 0.83 (0.73– 0.94) |
12,171 patients
117,851 HD patients
CV, cardiovascular; PD, peritoneal dialysis; HD, hemodialysis
Using HD patients with serum albumin between 4.00 and 4.19 g/dl at baseline as the reference group. Data adjusted for albumin category by modality, quarter of entry into study cohort, age, gender, race and/or ethnicity, diabetes, dialysis vintage, primary insurance, marital status, ischemic heart disease, congestive heart failure, myocardial infarction, cardiac arrest, pericarditis, cardiac dysrythmia, cerebrovascular disease, peripheral vascular disease, current smoking, body mass index, serum total iron binding capacity, ferritin, creatinine, calcium, phosphorus, parathyroid hormone, alkaline phosphatase, hemoglobin, white blood cell count, and % lymphocyte
Figure 3.
Comparison of survival predictability of baseline serum albumin in the unmatched cohort of patients treated with peritoneal dialysis and hemodialysis. Reference group: hemodialysis patients with serum albumin between 4.00 and 4.19 g/dl
Sensitivity Analysis
Almost identical results were obtained when the relationship of baseline, and time-averaged serum albumin, and change over time with all-cause, cardiovascular, and infection-related mortality was examined in the sub-group of patients who were being treated with PD at the time of entry into the study cohort. The comparison of survival predictability of serum albumin in PD and HD patients was repeated in the matched cohort, and the same trends were observed with a lower adjusted risk for death for patients treated with PD for each albumin category (Table S2).
Discussion
This analysis of data from a large, nationally representative and contemporary cohort of patients allows us to make several important observations. First, this study validates the prognostic value of serum albumin in PD patients. Second, to our knowledge this is the first demonstration that hypoalbuminemia predicts an increased risk of not only cardiovascular but also infection-related mortality in PD. Third, small changes in serum albumin over six months provide greater predictive value than that obtained from baseline serum albumin alone. Finally, for every serum albumin category, the relative risk of all-cause and cardiovascular mortality is lower for PD patients than those treated with HD.
Our analysis validates the predictive value of serum albumin in PD patients. More importantly, our study demonstrates that a low serum albumin is associated with increase in both cardiovascular and infection-related mortality. On one hand, the association of hypoalbuminemia with cardiovascular mortality in PD patients is similar to what has often been shown for HD patients 5, 12, 13. On the other hand, data demonstrating an association of low serum albumin to infection-related mortality are substantially more limited. Several studies have previously demonstrated an association between serum albumin at the time of start of PD with the subsequent risk for peritonitis 8, 14. However, the risk for death during episodes of peritonitis is < 5%, and most of the peritonitis-related deaths are secondary to cardiovascular causes, and not from overwhelming infection 15, 16. In contrast, over two-thirds of infection-related deaths in PD patients are attributed to “septicemia”, with pulmonary infections being the second most common cause 17. Furthermore, PD-related peritonitis is a local infection and rarely, if ever, leads to systemic infection. Hence, it is unlikely that the association of hypoalbuminemia with infection-related mortality in PD patients is secondary to the confounding influence of its association with episodes of peritonitis, and is a novel finding.
Another important, and heretofore under-appreciated, finding is that change in serum albumin over time provides prognostic information for PD patients over and above that which is obtained from the baseline serum albumin. Hence, patients in whom serum albumin declines by as little as 0.2 g/dl have a higher risk for death, irrespective of the baseline serum albumin. Our findings are consistent with another study that evaluated the relationship of change in serum albumin over time with outcome of PD patients 18. The current study also demonstrated that increases in serum albumin as little as 0.3 g/dl are associated with a lower risk for death for PD patients. This magnitude of change is either similar or smaller than what some studies have shown to be feasible with the administration of oral supplements or other therapeutic interventions (e.g., treatment of gastroparesis) in PD patients 1, 7. Hence, it is conceivable that therapeutic interventions that increase serum albumin may reduce the high risk of death of dialysis patients.
The biological basis for the association of hypoalbuminemia with all-cause, cardiovascular, and infection-related mortality remains uncertain. Since albumin is a negative acute phase reactant, it is widely believed that this association in dialysis patients is secondary to the confounding influence of systemic inflammation 19. A recent cross-sectional study of PD patients seems to suggest that many of the patients with hypoalbuminemia are volume overloaded and hypervolemia may be an additional confounding influence 20. There are two additional considerations unique to PD patients. First, PD patients lose 5–10 g/protein per day in the dialysate effluent, which is predominantly albumin 21. It is probably primarily for this reason that the serum albumin in PD patients is, on an average, lower than in HD patients 22, 23. The magnitude of daily peritoneal protein losses are thought to be a marker of systemic vascular disease burden and several recent studies have shown a direct relationship between daily peritoneal protein losses or clearance and all-cause mortality 24, 25. Our findings are consistent with these observations. Second, peritoneal protein losses are substantially increased during episodes of peritonitis and lead to significant decreases in serum albumin 26. As pointed out earlier, episodes of peritonitis are associated with a higher cardiovascular risk and this may be one of the reasons underlying the association of decrease in serum albumin with higher mortality in PD patients.
One of the most significant findings of this study was a demonstration that for every category of serum albumin, the risk for all-cause and cardiovascular mortality is lower in PD patients than in those treated with HD. In this study, while 37% of PD patients had a serum albumin ≥ 3.8 g/dl, 46% of HD patients met this threshold. An even larger difference was reported in the 2008 Clinical Performance Measures report – in a random sample of dialysis patients in the United States, whereas only 62% of PD patients had serum albumin ≥ 3.5 g/dl (n=1497), 82% of HD patients met this threshold (n=8,296)3. Systemic inflammation and low nutrient intake are thought to be the two most important contributors to hypoalbuminemia in dialysis patients 27. There is no evidence that a higher prevalence of either of these two abnormalities accounts for the higher prevalence of hypoalbuminemia in PD patients. Instead, it is likely the obligatory peritoneal protein losses with the therapy that account for the lower serum albumin seen in PD patients 22, 23. To our knowledge, this is the first study to directly compare the risk for death for a given range of serum albumin in PD and HD patients and it suggests that the decrease in serum albumin possibly related to peritoneal protein losses may not impose a higher risk for adverse events. Investigators from the NECOSAD (Netherlands Cooperative Study on Adequacy of Dialysis) recently reported similar findings – while every 1 g/dl lower serum albumin was associated with an adjusted hazard ratio of 1.47 for HD patients, the corresponding value for PD patients was 1.37 19. In our cohort, the risk for infection-related mortality was higher in every category of serum albumin for PD patients, a finding consistent with that recently reported from the Australia and New Zealand registry data28. Notwithstanding the higher risk of death from a single, albeit important, cause, the overall risk for death in each category of serum albumin was lower for PD patients. These findings indicate that the threshold for intervention, or considered adequate for health for dialysis patients, varies by dialysis modality. An inspection of our results suggest that the serum albumin that identifies an individual with a level of risk similar to that seen in HD patients, is lower by about 0.2–0.3 g/dl for patients treated with in PD.
Despite its considerable strengths, our study is not without its limitations. First, information on peritoneal transport rate, residual kidney function, PD prescription, or modality (continuous ambulatory or automated PD) was not available. However, it is unlikely that the lack of availability of these data biased our results. There is no evidence that that small solute clearances within the range achieved in clinical practice, have any effect on patient outcomes 29, 30. Furthermore, several large studies have demonstrated that there is no difference in the outcome of patients treated with either continuous ambulatory or automated PD 31, 32. Second, information on co-morbidity was obtained at the time of initiation of dialysis – 16–22 months from the time of entry into the study cohort. Third, information on inter-current events and/or interventions that may have led to changes in serum albumin was not available. Fourth, we cannot exclude the possibility of residual confounding. Patients with higher serum albumin, and in whom serum albumin increases over time are generally a healthier group of patients. Furthermore, PD patients are generally healthier than HD patients and the differences in health may not have been adequately captured by the available information on co-existing illnesses. However, this further strengthens our argument that the threshold level of serum albumin for intervention should be lower for PD patients than those treated with HD. Fifth, there were no data available on serum levels of acute phase reactants or cytokines. However, we included several surrogate measures like total iron binding capacity, ferritin, and total lymphocyte count which are arguably are more relevant since they are routinely measured for all dialysis patients in the United States.
To conclude, in this large and contemporary cohort of dialysis patients, we validated the prognostic value of serum albumin in PD patients. Moreover, periodic measurement of serum albumin provides information over and above what is obtained from a single measurement. Finally, the level of serum albumin that mandates clinical evaluation, and intervention and is used by regulators to judge the quality of care should vary by dialysis modality. Hence, this threshold for PD patients should be 0.2–0.4 g/dl lower than for HD patients.
Supplementary Material
Association of time-averaged serum albumin concentration with outcomes in patients undergoing peritoneal dialysis (n=12,171). 2A) all-cause mortality; 2B) cardiovascular mortality; and 2C) infection-related mortality. Reference group: peritoneal dialysis patients with serum albumin between 4.00 and 4.19 g/dl.
Acknowledgements
Support: The work in this manuscript is supported by grants from the NIH (DK077341), and DaVita Inc. to Drs Mehrotra and Kalantar-Zadeh.
Financial Disclosure: Dr Mehrotra has received research grants, served as ad hoc consultant, and honoraria from Baxter Health Care. Drs Mehrotra and Kalantar-Zadeh have received research grants from DaVita Inc. Dr Moran is an employee of DaVita Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Table S1. Crude outcomes of patients based upon baseline serum albumin, dialysis modality, and vintage.
Table S2. Association of baseline serum albumin and all-cause and CV mortality in a matched cohort of patients treated with PD and HD.
Figure S1: Association of time-averaged serum albumin concentration in PD patients with all-cause mortality, CV mortality, and infection-related mortality.
Note: The supplementary material accompanying this article (doi: ________ ) is available at www.ajkd.org.
Descriptive Text for Online Delivery
Hyperlink: Supplementary Table S1 (PDF)
About: Crude outcomes of patients based upon baseline serum albumin, dialysis modality, and vintage.
Hyperlink: Supplementary Table S2 (PDF)
About: Association of baseline serum albumin and all-cause and cardiovascular mortality in a matched cohort of patients treated with peritoneal dialysis (n=8,500) and hemodialysis (n=8,500).
Hyperlink: Supplementary Figure S1 (PDF)
About: Association of time-averaged serum albumin concentration with outcomes in patients undergoing peritoneal dialysis (n=12,171). Outcomes studied are A) all-cause mortality, B) cardiovascular mortality, and C) infection-related mortality. Reference group: peritoneal dialysis patients with serum albumin between 4.00 and 4.19 g/dL.
References
- 1.Kalantar-Zadeh K, Budde K, Cano N, et al. Meal and enteral supplements for improving nutritional status and outcomes in chronic kidney disease. Nat Rev Nephrol. 2010 doi: 10.1038/nrneph.2011.60. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocco MV, Frankenfield DL, Hopson SD, McClellan WM. Relationship between clinical performance measures and outcomes among patients receiving long-term hemodialysis. Annals of internal medicine. 2006 Oct 3;145(7):512–519. doi: 10.7326/0003-4819-145-7-200610030-00009. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services. 2008 Annual Report, End Stage Renal Disease Clinical Performance Measures Project. Baltimore, Maryland: Department of Health and Human Services, Center for Medicare and Medicaid Services, Office of Clinical Standards and Quality; 2008. Dec, [Google Scholar]
- 4.Mehrotra R, Kopple JD. Nutritional management of maintenance dialysis patients: why aren't we doing better? Annu Rev Nutr. 2001;21:343–379. doi: 10.1146/annurev.nutr.21.1.343. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, et al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant. 2005 Sep;20(9):1880–1888. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999 Mar;33(3):523–534. doi: 10.1016/s0272-6386(99)70190-3. [DOI] [PubMed] [Google Scholar]
- 7.Mehrotra R, Kopple JD. Protein and energy nutrition among adult patients treated with chronic peritoneal dialysis. Adv Ren Replace Ther. 2003 Jul;10(3):194–212. doi: 10.1053/j.arrt.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Bernardini J, Piraino B, Fried L. Albumin at the start of peritoneal dialysis predicts the development of peritonitis. Am J Kidney Dis. 2003 Mar;41(3):664–669. doi: 10.1053/ajkd.2003.50128. [DOI] [PubMed] [Google Scholar]
- 9.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar Outcomes With Hemodialysis and Peritoneal Dialysis in Patients With End-Stage Renal Disease. Arch Intern Med. 2011 Jan 24;171(2):110–118. doi: 10.1001/archinternmed.2010.352. [DOI] [PubMed] [Google Scholar]
- 10.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010 Mar;21(3):499–506. doi: 10.1681/ASN.2009060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu YW, Jiwakanon S, Lukowsky L, Duong U, Kalantar-Zadeh K, Mehrotra R. An Update on the Comparisons of Mortality Outcomes of Hemodialysis and Peritoneal Dialysis Patients. Semin Nephrol. 2011 doi: 10.1016/j.semnephrol.2011.01.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol. 1996 May;7(5):728–736. doi: 10.1681/ASN.V75728. [DOI] [PubMed] [Google Scholar]
- 13.Molnar MZ, Kovesdy CP, Bunnapradist S, et al. Associations of Pretransplant Serum Albumin with Post-Transplant Outcomes in Kidney Transplant Recipients. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03480.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirivongs D, Pongskul C, Keobounma T, Chunlertrith D, Sritaso K, Johns J. Risk factors of first peritonitis episode in Thai CAPD patients. J Med Assoc Thai. 2006 Aug;89 Suppl 2:S138–S145. [PubMed] [Google Scholar]
- 15.Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl. 2006 Nov;(103):S55–S62. doi: 10.1038/sj.ki.5001916. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Fontan M, Rodriguez-Carmona A, Garcia-Naveiro R, Rosales M, Villaverde P, Valdes F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25:274–284. [PubMed] [Google Scholar]
- 17.United States Renal Data System. US Department of Public Health and Human Services. Bethesda: Public Health Service, National Institutes of Health; 2009. [Google Scholar]
- 18.Jones CH, Newstead CG, Wills EJ, Davison AM. Serum albumin and survival in CAPD patients: the implications of concentration trends over time. Nephrol Dial Transplant. 1997 Mar;12(3):554–558. doi: 10.1093/ndt/12.3.554. [DOI] [PubMed] [Google Scholar]
- 19.de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009 Mar;19(2):127–135. doi: 10.1053/j.jrn.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 20.John B, Tan BK, Dainty S, Spanel P, Smith D, Davies SJ. Plasma volume, albumin, and fluid status in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2010 Aug;5(8):1463–1470. doi: 10.2215/CJN.09411209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westra WM, Kopple JD, Krediet RT, Appell M, Mehrotra R. Dietary protein requirements and dialysate protein losses in chronic peritoneal dialysis patients. Perit Dial Int. 2007 Mar-Apr;27(2):192–195. [PubMed] [Google Scholar]
- 22.Yeun JY, Kaysen GA. Acute phase proteins and peritoneal dialysate albumin loss are the main determinants of serum albumin in peritoneal dialysis patients. Am J Kidney Dis. 1997 Dec;30(6):923–927. doi: 10.1016/s0272-6386(97)90105-0. [DOI] [PubMed] [Google Scholar]
- 23.Heaf JG, Sarac S, Afzal S. A high peritoneal large pore fluid flux causes hypoalbuminaemia and is a risk factor for death in peritoneal dialysis patients. Nephrol Dial Transplant. 2005 Oct;20(10):2194–2201. doi: 10.1093/ndt/gfi008. [DOI] [PubMed] [Google Scholar]
- 24.Szeto CC, Chow KM, Lam CWK, et al. Peritoneal albumin excretion is a strong predictor of cardiovascular events in peritoneal dialysis patients: a prospective cohort study. Perit Dial Int. 2005;25:445–452. [PubMed] [Google Scholar]
- 25.Perl J, Huckvale K, Chellar M, John B, Davies SJ. Peritoneal protein clearance and not peritoneal membrane transport status predicts survival in a contemporary cohort of peritoneal dialysis patients. Clin J Am Soc Nephrol. 2009 Jul;4(7):1201–1206. doi: 10.2215/CJN.01910309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenkrantz MJ, Gahl GM, Kopple JD, et al. Protein losses during peritoneal dialysis. Kidney Int. 1981;19:593–602. doi: 10.1038/ki.1981.57. [DOI] [PubMed] [Google Scholar]
- 27.Kaysen GA, Chertow GM, Adhikarla R, Young B, Ronco C, Levin NW. Inflammation and dietary protein intake exert competing effects on serum and albumin, creatinine in hemodialysis patients. Kidney Int. 2001;60:333–340. doi: 10.1046/j.1523-1755.2001.00804.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson DW, Dent H, Hawley CM, et al. Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am J Kidney Dis. 2009 Feb;53(2):290–297. doi: 10.1053/j.ajkd.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 29.Paniagua R, Amato D, Vonesh E, et al. Effects of Increased Peritoneal Clearances on Mortality Rates in Peritoneal Dialysis: ADEMEX, a Prospective, Randomized, Controlled Trial. J Am Soc Nephrol. 2002 May 1;13(5):1307–1320. doi: 10.1681/ASN.V1351307. 2002. [DOI] [PubMed] [Google Scholar]
- 30.Lo WK, Ho YW, Li CS, et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int. 2003;64:649–656. doi: 10.1046/j.1523-1755.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 31.Badve SV, Hawley CM, McDonald SP, et al. Automated and continuous ambulatory peritoneal dialysis have similar outcomes. Kidney Int. 2008 Feb;73(4):480–488. doi: 10.1038/sj.ki.5002705. [DOI] [PubMed] [Google Scholar]
- 32.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Vonesh E. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int. 2009 Jul;76(1):97–107. doi: 10.1038/ki.2009.94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association of time-averaged serum albumin concentration with outcomes in patients undergoing peritoneal dialysis (n=12,171). 2A) all-cause mortality; 2B) cardiovascular mortality; and 2C) infection-related mortality. Reference group: peritoneal dialysis patients with serum albumin between 4.00 and 4.19 g/dl.