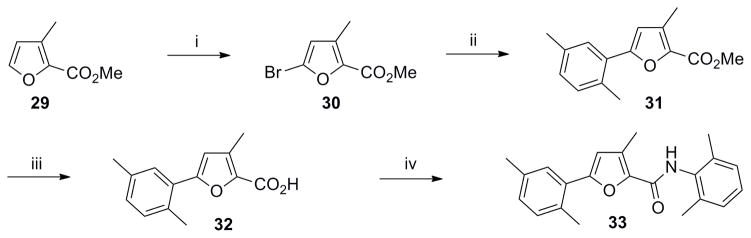
Scheme 7. Synthesis of 2,3,5-trisubstituted furan analog 33.
Reagents and conditions: (i) NBS (1.1 equiv), MeOH/THF, 0 °C to rt, 2 h, 60%; (ii) Pd(OAc)2 (0.05 equiv), Cy3P (0.01 equiv), 16 (1.4 equiv), K3PO4 (3 equiv), toluene/H2O (8:1), 100 °C, 12 h, 50%; (iii) LiOH (1.3 equiv), THF/MeOH/H2O (2: 2:1), rt, 3 h; (iv) (a) (COCl)2 (1.3 equiv), DMF, CH2Cl2, rt, overnight: (b) Et3N (2 equiv), 4 (1.5 equiv), CH2Cl2, rt, overnight, 80% ( over 3 steps).