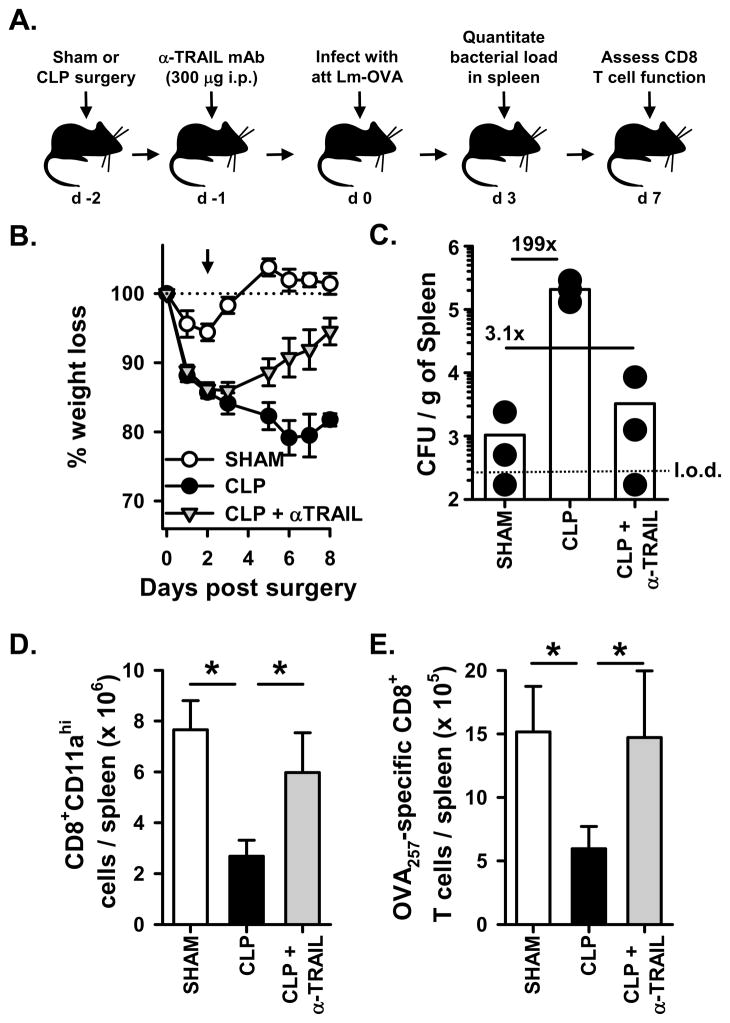
Figure 6.
Administration of an anti-TRAIL blocking mAb to WT B6 mice after CLP treatment lessens morbidity, increases control of the bacterial infection, and restores CD8 T cell responses. A. Experimental design. Sham or CLP surgery was performed on WT B6 mice, and some of the CLP-treated mice received the blocking anti-TRAIL mAb N2B2 (300 μg i.p.) on d 1 post-surgery. On d 2 post-surgery, the mice were infected with 107 CFU attenuated LM-OVA. B. Animal weight was measured daily beginning the day of surgery, and the percent weight loss with respect to the starting weight is shown. The dotted line represents the starting weight of the mice, normalized to 100%, and the arrow indicates the time of infection. C. The bacterial titer in the spleen was determined on d 3 post-infection. The dotted line represents the limit of detection (l.o.d.) for bacterial count in the spleen. On d 7 after infection, the number of Ag-experienced (D) and OVA257-specific (E) CD8 T cells in the spleen was determined. *p < 0.05 (ANOVA). There was no significant difference between the Sham and CLP + anti-TRAIL mAb groups.