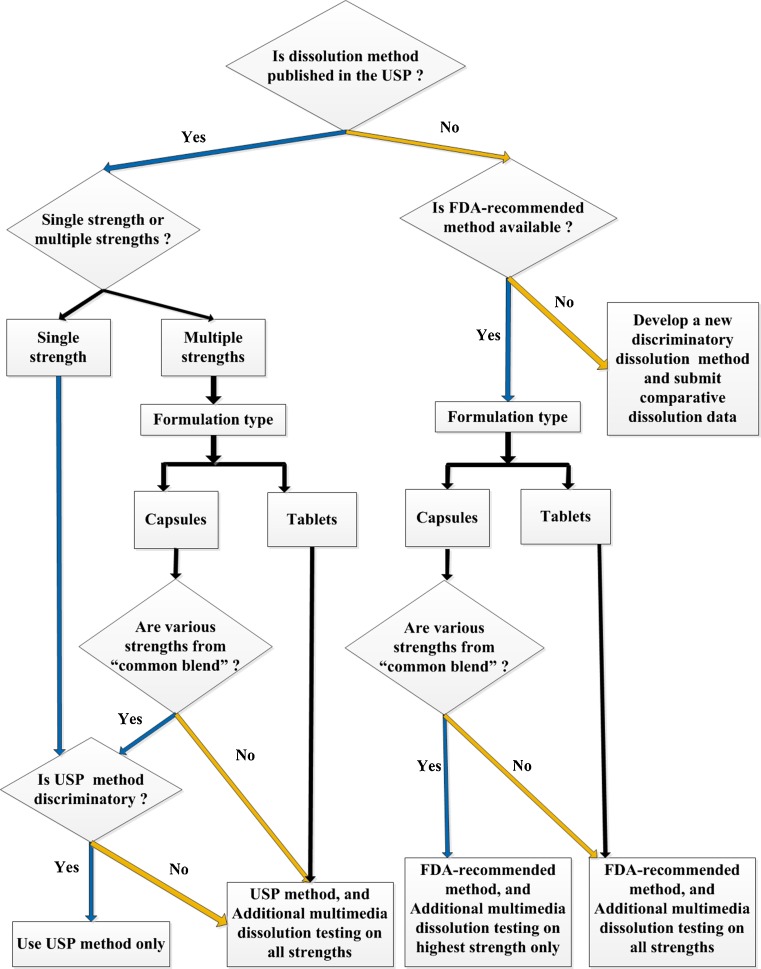
Fig. 2.
Decision-tree for ANDA sponsors for submitting dissolution testing data for an extended-release solid oral generic drug product. {“Common blend”: A batch of final blend that can be packed in different amounts providing various strengths of the capsule product. Multimedia: lower pH, e.g. 1.2; medium pH, e.g. 4.5; higher pH, e.g. 6.8 and water}