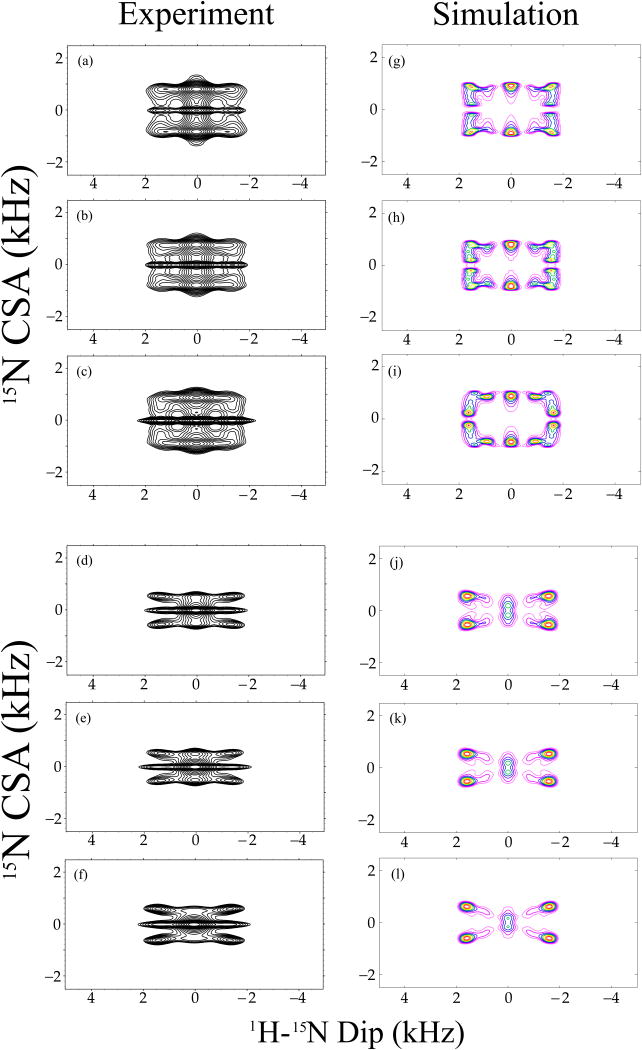
Figure 10.
Experimental (a-f) and simulated (g-l) 15N CSA/1H-15N dipolar correlation spectra of 13C, 15N N-formyl-Met-Leu-Phe (MLF) tripeptide, extracted from the corresponding 3D experiments along the 15N isotropic chemical shift for Phe (a, d), Leu (b, e) and Met (c, f) residues. 3D R1817/R1425 and R1817/R1423 pulse sequences were used to record the experimental (a - c) and (d - f) spectra, respectively, and the same sequences were employed to generate the simulated (g – i) and (j – l) spectra, respectively. The sample was spun at a MAS frequency of 10 kHz. 1H-15N dipolar and 15N CSA parameters used in the simulations were extracted from 1D R18 dipolar and 1D R14 CSA pattern simulations, and fixed during the calculations. The orientation of the 15N CSA relative to 1H-15N dipolar tensor was optimized. The best-fit relative orientations between the 1H-15N dipolar and 15N CSA are shown in Table 2.