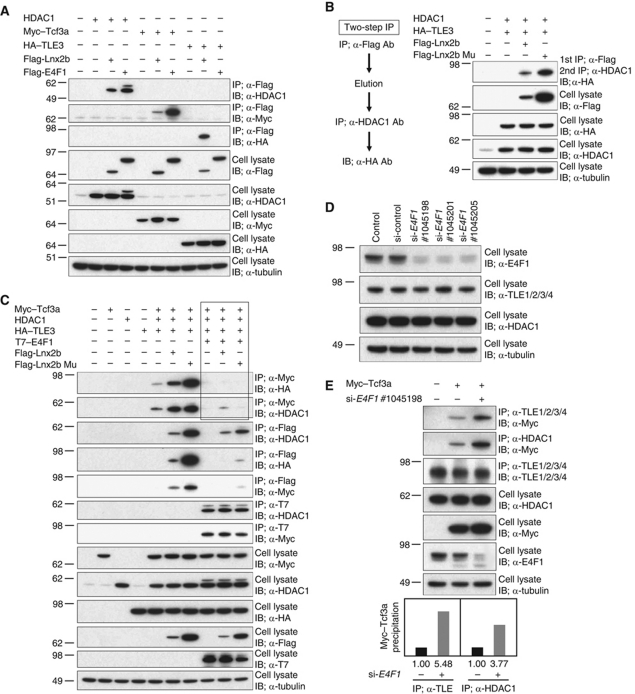
Figure 6.
E4f1 and Lnx2b modulate binding of Tcf3 to corepressor molecules. (A–E) 293T cells were transfected with the indicated plasmids. (A) Co-IP at 48 h post-transfection and blotting was carried out with the antibodies indicated. Lnx2b co-precipitated with Tcf3, TLE3 and HDAC1, while E4f1 co-precipitated with HDAC1 and Tcf3 but not with TLE3. Note that Lnx2b mutant accumulated to a higher level than WT due to self-ubiquitylation and destabilization of the latter (Ro and Dawid, 2009). (B) Two-Step IP. Lysates of cells transfected with plasmids encoding HDAC1 and HA-tagged TLE3, plus Flag-Lnx2b or Flag-Lnx2b Mu, were first precipitated with anti-Flag antibody. Proteins eluted by 3xFlag peptide were subjected to a second IP with anti-HDAC1 antibody; co-precipitated TLE3 was detected by anti-HA blotting. (C) Lnx2b, WT or mutant, enhanced co-precipitation yields of Tcf3a with HDAC1 or TLE3. By contrast, E4f1 overexpression dissociated the corepressors and WT or Mu Lnx2b from Tcf3a; the lanes showing this critical observation are outlined in the figure. Association between E4f1 and Tcf3a and between E4f1 and HDAC1 was not altered by the presence of Lnx2b, and the association between Lnx2b/Lnx2b Mu and HDAC1 was not greatly altered by E4f1. (D) All three E4F1 siRNAs tested knocked downed E4F1 levels. (E) Depletion of E4F1 facilitated binding of endogenous HDAC1 and TLE family proteins to Tcf3a. The histogram at the bottom shows quantification of this experiment using ImageJ.