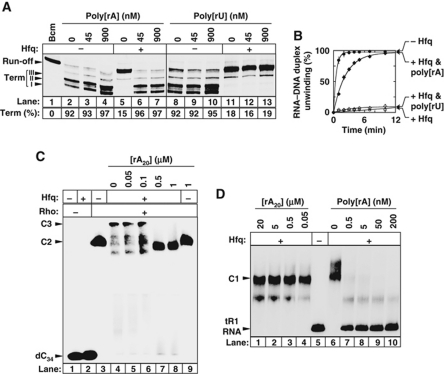
Figure 7.
Interaction with the distal, polyA-binding face of Hfq is critical for Rho inhibition. (A) Effect of polynucleotides (concentrations in nucleotides are indicated above gel lanes) on Hfq-mediated transcription antitermination at λtR1 in the presence of 70 nM Rho. In vitro transcriptions were performed with the pT7A1-λcro template (Figure 2A) and Hfq (+ lanes) present at a concentration of 210 nM. Global termination efficiencies (Term) are indicated below gel lanes. (B) Effect of the polynucleotides (900 nM, in nucleotides) on Rho-directed unwinding of the tR1RNA/D21 duplex. The concentrations of Rho and Hfq (in selected samples) were 20 nM and 210 nM, respectively. (C) Oligomer rA20 prevents the association of Hfq with the binary Rho–dC34 complex. Assay conditions are as in Figure 5B. Concentration of Hfq was 375 nM. (D) Oligomer rA20 does not displace Hfq from tR1RNA whereas poly[rA] does.