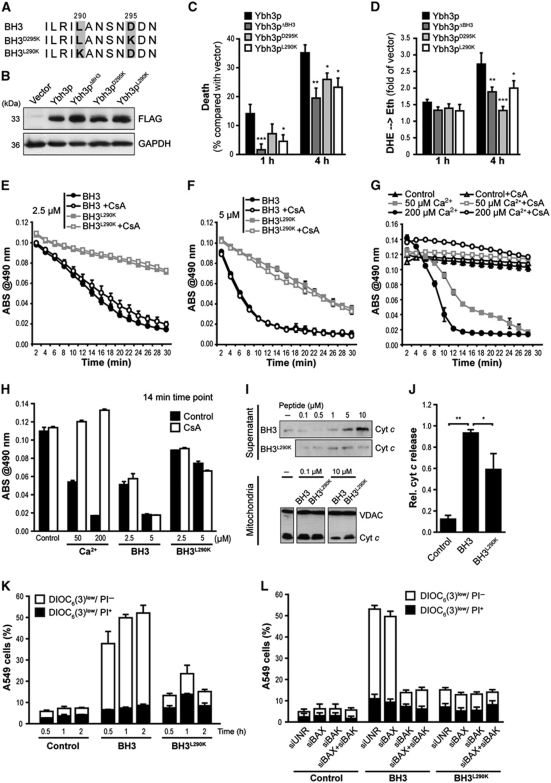
Figure 3.
The yeast BH3 domain induces apoptotic changes in isolated mitochondria as well as in mammalian and yeast cells. (A) Amino-acid sequence alignment of the native yeast BH3 domain and the BH3D295K and BH3L290K point mutants. (B) Immunoblot analysis of WT yeast cells for FLAG-tagged Ybh3p, Ybh3pΔBH3, Ybh3pD295K or Ybh3pL290K. (C, D) Death determined by clonogenicity (C) and quantification of ROS accumulation using DHE → Eth conversion (D) of yeast cells overexpressing Ybh3p, Ybh3pΔBH3, Ybh3pD295K or Ybh3pL290K after treatment with 120 mM acetic acid for 1 or 4 h. Death rates were calculated by setting the survival of vector control cells to 100% and thus 0% death (mean±s.e.m., n=8). (E–H) Swelling of mouse liver mitochondria as indicated by loss of absorbance (ABS) at 490 nm. Mitochondria were pre-incubated or not with 5 μM CsA, followed by the administration of 2.5 μM (E) or 5 μM (F) of native (BH3) or mutated (BH3L290K) peptide or indicated concentrations of Ca2+(G). To permit direct comparison, swelling after 14 min under all conditions analysed was depicted in (H). Representative experiments are shown, with data representing mean±s.e.m. of three replicates. (I, J) Immunoblot analysis of supernatants and mitochondrial pellets (I) from mouse liver mitochondria incubated with the indicated concentration of native (BH3) or mutated (BH3L290K) peptide using an antibody specific for cyt c. For supernatants, the signal intensities of cyt c were quantified and normalized to VDAC intensities of deployed mitochondria (J) (mean±s.e.m., n=3). (K) Non-small cell lung cancer (A549) cells were incubated with 30 μM of the native (BH3) or mutated (BH3L290K) peptide, followed by the flow cytometric assessment of Δψm dissipation (DiOC6(3)low) and loss of plasma membrane integrity (PI+) (mean±s.e.m., n=5). (L) A549 cells transfected with a control siRNA (siUNR) or with siRNA targeting BAX, BAK or with a combination of these were treated with 30 μM of the native (BH3) or mutated (BH3L290K) peptide followed by the flow cytometric assessment of Δψm dissipation (DiOC6(3)low) and loss of plasma membrane integrity (PI+). Data represent mean±s.e.m. and one representative experiment is shown with n=3. *P<0.05, **P<0.01 and ***P<0.001.