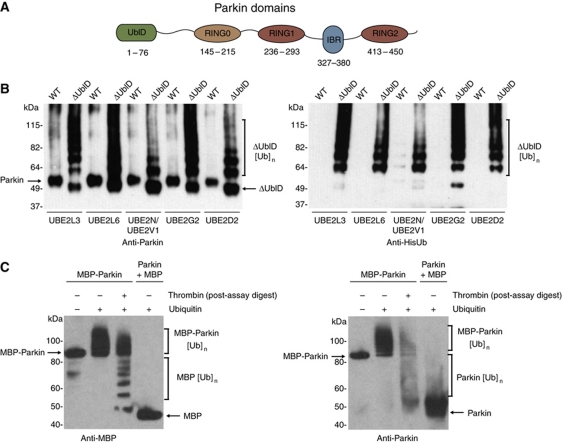
Figure 1.
The ubiquitin-like domain of Parkin inhibits its autoubiquitination. (A) Schematic representation of Parkin molecule. The ubiquitin-like (UblD), RING0 (R0), RING (R1, R2) and in-between-RING (IBR) domains are indicated. The numbering for each domain is as per the human protein. (B) Western blot analysis of autoubiquitination assays of WT Parkin (WT) and ΔUbl-Parkin (ΔUblD) reveal inhibition of Parkin autoubiquitination by the Ubl domain. Formation of ubiquitin conjugates is detected with α-Parkin (left) and α-His-Ub (right) and indicated with a bracket. The E2 used in each experiment is indicated. (C) Western blot analysis of autoubiquitination assays of MBP-Parkin, showing competence for autoubiquitination only in the fused protein. Thrombin was added after the reaction has been stopped, leading to the cleavage of ubiquitinated MBP-Parkin. Detection of conjugates by MBP and Parkin-specific antibodies reveals that most of the ubiquitin moieties are on the MBP tag (lane 3). Lane 1 has no ubiquitin. Brackets indicate ubiquitination.